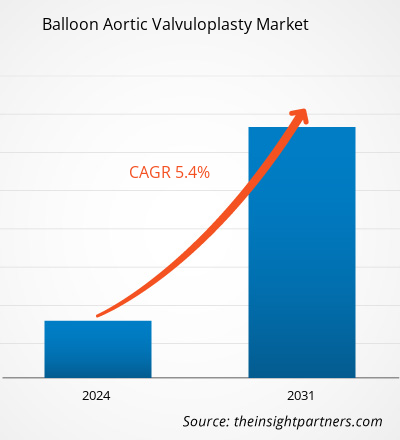
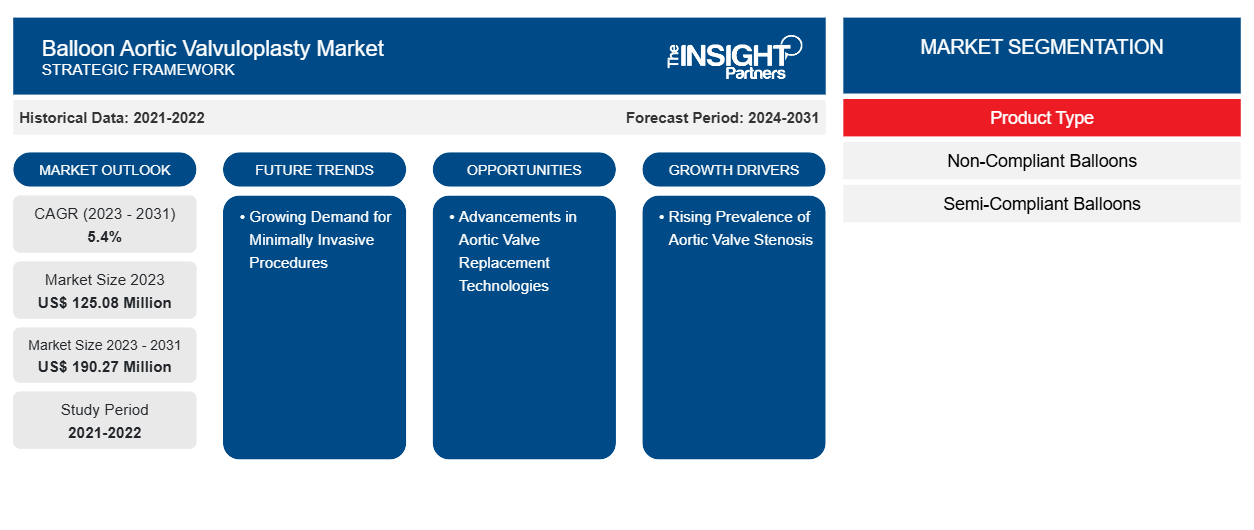
من المتوقع أن يصل حجم سوق رأب الصمام الأورطي بالبالون إلى 190.27 مليون دولار أمريكي بحلول عام 2031 من 125.08 مليون دولار أمريكي في عام 2023. ومن المتوقع أن يسجل السوق معدل نمو سنوي مركب بنسبة 5.4٪ خلال الفترة 2023-2031. ومن المرجح أن تظل استراتيجيات البحث والتطوير الناشئة التي تساعد في تحسين زراعة الصمام الأورطي عبر القسطرة (TAVI) من الاتجاهات الرئيسية في السوق.
تحليل سوق جراحة توسيع الصمام الأورطي بالبالون
تشمل العوامل التي تدفع نمو سوق أجهزة رأب الصمامات بالبالون الشيخوخة السريعة للسكان ومعدل انتشار تضيق الصمام الأبهري المتزايد. أصبح تضيق الصمام الأبهري أكثر أمراض الصمامات شيوعًا والتي تتطلب علاجًا جراحيًا. يمكن أن تكون عملية رأب الصمام الأبهري بالبالون مفيدة في مجموعة واسعة من المرضى الذين يعانون من تضيق الأبهر، بالإضافة إلى كونها علاجًا مؤقتًا لاستبدال الصمام، إلى جانب دورها في إجراءات TAVI. إن التوافر المتزايد للتعويضات الطبية لأجهزة رأب الصمامات بالبالون، والتبني المتزايد للجراحات الأقل توغلاً، والبنية التحتية للرعاية الصحية الأفضل هي عوامل إضافية تساهم في نمو السوق. تتضمن الأساليب الأقل توغلاً عادةً شقوقًا أصغر باستخدام أدوات وتقنيات متخصصة للوصول إلى القلب، مما قد يؤدي إلى تقصير مدة الإقامة في المستشفى وتقليل الندوب. بالإضافة إلى ذلك، تستثمر مؤسسات الرعاية الصحية في تدريب فرقها الطبية والحصول على المعدات اللازمة لتلبية الطلب المتزايد على جراحات الصمام الأورطي بالبالون وقسطرة القسطرة الأبهرية الأقل توغلاً، وبالتالي توفير خيارات علاجية محسنة للمرضى وتجارب رعاية صحية أفضل بشكل عام. وبالتالي، تشهد سوق جراحة الصمام الأورطي بالبالون نموًا ملحوظًا.
نظرة عامة على سوق جراحة توسيع الصمام الأورطي بالبالون
يموت أكثر من نصف المرضى الذين يعانون من أمراض صمام القلب الشديدة في غضون عامين من ظهور الأعراض. تشير نتائج المسح التي نشرتها Global Heart Hub في Heart Valve Voice Canada 2020 إلى أن 3٪ فقط من الكنديين الذين تبلغ أعمارهم 60 عامًا أو أكثر يدركون تضيق الأبهر. لذلك فإن زيادة الوعي والكشف المبكر عن أمراض صمام القلب أمر ضروري للمرضى، إلى جانب أولئك المعتمدين عليهم والمجتمع المحلي. شكلت الجمعية الكندية لأمراض القلب والأوعية الدموية مجموعة عمل استراتيجية أمراض صمام القلب لمعالجة الثغرات في رعاية أمراض صمام القلب في كندا. في يناير 2021، تلقت Medtronic إشارة موسعة من Health Canada لنظام Evolut TAVI. منصة TAVI من Medtronic هي النظام الوحيد المرخص لكل من صمامات الأبهر ثنائية الشرفات (المرضى المعرضين لخطر متوسط أو أعلى). يُشار إلى Evolut في البلاد لمرضى تضيق الأبهر الشديد في جميع فئات المخاطر. في سبتمبر 2021، حصلت شركة أبوت على موافقة إدارة الغذاء والدواء الأمريكية (FDA) على نظام Portico مع نظام استبدال الصمام الأورطي عبر القسطرة (TAVR) FlexNav لعلاج الأشخاص الذين يعانون من تضيق الأبهر الشديد المصحوب بأعراض والذين هم معرضون لخطر كبير لإجراء جراحة القلب المفتوح.
قم بتخصيص هذا التقرير ليناسب متطلباتك
ستحصل على تخصيص لأي تقرير - مجانًا - بما في ذلك أجزاء من هذا التقرير، أو تحليل على مستوى الدولة، وحزمة بيانات Excel، بالإضافة إلى الاستفادة من العروض والخصومات الرائعة للشركات الناشئة والجامعات
-
احصل على أهم اتجاهات السوق الرئيسية لهذا التقرير.ستتضمن هذه العينة المجانية تحليلاً للبيانات، بدءًا من اتجاهات السوق وحتى التقديرات والتوقعات.
محركات وفرص سوق جراحة توسيع الصمام الأورطي بالبالون
الطلب المتزايد على الإجراءات الجراحية الأقل تدخلاً يعزز نمو السوق
يعكس الطلب المتزايد على التقنيات الأقل تدخلاً في جراحات استبدال الصمامات تحولاً كبيراً في الممارسات الطبية وتفضيلات المرضى. في عملية رأب الصمام الأورطي الأقل تدخلاً، يتم إصلاح الصمام الأورطي للقلب واستبداله إذا لزم الأمر من خلال إجراءات TAVI. يتم دفع التقنيات الأقل تدخلاً برغبة في تقليل التدخل الجراحي، وتقليل المخاطر المرتبطة بها، وتعزيز أوقات التعافي بشكل أسرع.
تشهد جميع فروع الطب، بما في ذلك أمراض القلب، زيادة في الطلب على الإجراءات الأقل توغلاً بسبب العديد من الفوائد مقارنة بجراحة القلب المفتوح التقليدية. بالمقارنة مع جراحة القلب المفتوح، فإن المرضى الذين يخضعون لإجراءات طفيفة التوغل لديهم عادةً وقت تعافي أقصر. بالإضافة إلى ذلك، فإن التقنيات الأقل توغلاً لها مخاطر أقل للمضاعفات وتؤدي إلى ندوب أقل مقارنة بجراحة القلب المفتوح. وقد تبنى الجراحون هذه التقنيات بسبب التقدم في الأجهزة الطبية، ويمكن أن تسهل المهارات الجراحية المحسنة التبني على نطاق واسع. نظرًا لأن BAV هي تقنية قليلة التوغل يمكن استخدامها لعلاج مجموعة متنوعة من مشاكل القلب، بما في ذلك تضيق الصمام الأورطي ، فإن سوق أجهزة BAV مدفوع بالطلب المتزايد على الإجراءات الأقل توغلاً.
التطورات في تقنيات استبدال الصمام الأورطي
يشعر المرضى الذين يعانون من أمراض القلب بتحسن في الأعراض خلال فترة أقصر عند علاجهم بإجراءات القسطرة مقارنة بالتدخلات الجراحية. وقد أدت التقنيات الجراحية الأقل توغلاً، مثل TAVI، إلى تحديث علاج تضيق الأبهر بسبب التقدم. بالإضافة إلى ذلك، أدت التحسينات في التصميم إلى تطوير بدائل صمام الأبهر الأقوى والمتوافقة حيوياً، والتي عززت نتائج المرضى وخفضت من خطر حدوث المضاعفات. فيما يلي ذكر بعض التطورات التكنولوجية التي حققتها الشركات في مجال رأب الصمام الأورطي بالبالون وأجهزة استبدال الصمام الأورطي:
- في يناير 2023، حصلت شركة أبوت على موافقة إدارة الغذاء والدواء الأمريكية على نظام Navitor TAVI لعلاج المرضى الذين يعانون من تضيق الأبهر الشديد وأولئك غير المؤهلين لجراحة القلب المفتوح. تم تقديم Navitor حديثًا في محفظة القلب الهيكلي عبر القسطرة الشاملة للشركة؛ فهو يوفر للأطباء والمرضى بدائل علاجية أقل تدخلاً لمجموعة من أمراض القلب الخطيرة.
- في سبتمبر 2020، قدمت شركة Boston Scientific نظام صمام الأبهر ACURATE neo2 في أوروبا. كانت تقنية TAVI من الجيل التالي - وهي منصة جديدة مصممة بميزات متعددة لتحسين الأداء السريري لمنصة ACURATE neo الأصلية - ذات مؤشر ممتد للمرضى الذين يعانون من تضيق الأبهر مقارنة بنظام صمام الأبهر من الجيل السابق.
لذلك، فإن التقدم التكنولوجي يوفر فرصًا مربحة لنمو سوق جراحة صمام الأبهر بالبالون.
تقرير تحليلي لتجزئة سوق جراحة رأب الصمام الأورطي بالبالون
إن القطاع الرئيسي الذي ساهم في اشتقاق تحليل سوق جراحة صمام الأبهر بالبالون هو نوع المنتج.
بناءً على نوع المنتج، ينقسم سوق رأب الصمام الأورطي بالبالون إلى بالونات غير متوافقة وبالونات شبه متوافقة. احتلت شريحة البالونات غير المتوافقة حصة سوقية أكبر في عام 2023 ومن المتوقع أن تسجل معدل نمو سنوي مركب أعلى في السوق خلال الفترة 2023-2031.
تحليل حصة سوق جراحة توسيع الصمام الأورطي بالبالون حسب المنطقة الجغرافية
ينقسم النطاق الجغرافي لتقرير سوق جراحة صمام الأبهر بالبالون بشكل أساسي إلى خمس مناطق: أمريكا الشمالية، وآسيا والمحيط الهادئ، وأوروبا، والشرق الأوسط وأفريقيا، وأمريكا الجنوبية والوسطى.
استحوذت أمريكا الشمالية على حصة كبيرة من السوق. ويعزى نمو السوق في هذه المنطقة إلى الانتشار المتزايد لأمراض صمامات القلب، وزيادة المبادرات من قبل المنظمات المختلفة لزيادة الوعي بأمراض صمامات القلب بين السكان، والتقدم المحرز في أجهزة العلاج. ووفقًا لمنظمة التعاون الاقتصادي والتنمية، فإن عبء أمراض القلب والأوعية الدموية يتزايد بسرعة في المكسيك. يتم التعامل مع عوامل الخطر المرتبطة بأمراض القلب بين السكان البالغين وكبار السن في المكسيك من خلال برامج التوعية. واستجابة لهذه البرامج التوعوية، اكتسبت جراحات استبدال الصمام الأورطي عن طريق القسطرة زخمًا ويتم تفضيلها بشكل عام كبديل لجراحة صمام القلب المفتوح. يقوم أطباء القلب في مدينة مكسيكو بإجراء TAVI باستخدام أفضل التقنيات والأساليب. تعد مستشفيات CMQ رائدة في إجراء TAVI في بويرتو فالارتا وريفييرا ناياريت. تضم المستشفيات فريقًا متعدد التخصصات من المتخصصين في أمراض القلب التداخلية، مما يضمن رعاية شاملة وشخصية لكل مريض.
السبب الأكثر شيوعًا لتضيق الأبهر في كندا هو مرض الصمام التنكسي بسبب الشيخوخة، يليه مرض الصمام الأورطي ثنائي الشرفات الخلقي وأمراض القلب الروماتيزمية. وفقًا لـ "مرض صمام القلب في كندا"، الذي نشره معهد اقتصاديات الصحة في مارس 2022، فإن تضيق الأبهر وقصور الصمام التاجي هما أكثر أمراض صمام القلب شيوعًا. أفادت التقارير أن حوالي 2.5٪ من سكان البلاد يعانون من أمراض صمام القلب، والتي تزداد بشكل كبير بعد سن 65 عامًا، لتصل إلى 13٪ لدى الأشخاص الذين تبلغ أعمارهم 75 عامًا أو أكثر. ومن المقدر أن يعاني 1.5 مليون شخص فوق سن 65 عامًا من أمراض صمام القلب بحلول عام 2040 في كندا.
في كندا، يقدم عدد كبير من اللاعبين في السوق منتجات TAVI. تعد Edwards Lifesciences وBoston Scientific Corporation وMedtronic Inc. وJenaValve Technology وTranscatheter Technologies GmbH من بين الشركات المصنعة القليلة العاملة في السوق. وقد حصلت هذه الشركات المصنعة على الموافقات التنظيمية من الحكومة الكندية. وقد حصلت أنظمة Edwards SAPIEN وMedtronic CoreValve على تراخيص في كندا.
رؤى إقليمية حول سوق جراحة توسيع الصمام الأورطي بالبالون
لقد قام المحللون في Insight Partners بشرح الاتجاهات والعوامل الإقليمية المؤثرة على سوق رأب الصمام الأورطي بالبالون طوال فترة التوقعات بشكل شامل. يناقش هذا القسم أيضًا قطاعات سوق رأب الصمام الأورطي بالبالون والجغرافيا في جميع أنحاء أمريكا الشمالية وأوروبا ومنطقة آسيا والمحيط الهادئ والشرق الأوسط وأفريقيا وأمريكا الجنوبية والوسطى.
- احصل على البيانات الإقليمية المحددة لسوق جراحة رأب الصمام الأورطي بالبالون
نطاق تقرير سوق جراحة رأب الصمام الأورطي بالبالون
| سمة التقرير | تفاصيل |
|---|---|
| حجم السوق في عام 2023 | 125.08 مليون دولار أمريكي |
| حجم السوق بحلول عام 2031 | 190.27 مليون دولار أمريكي |
| معدل النمو السنوي المركب العالمي (2023 - 2031) | 5.4% |
| البيانات التاريخية | 2021-2022 |
| فترة التنبؤ | 2024-2031 |
| القطاعات المغطاة |
حسب نوع المنتج
|
| المناطق والدول المغطاة |
أمريكا الشمالية
|
| قادة السوق وملفات تعريف الشركات الرئيسية |
|
كثافة اللاعبين في سوق جراحة توسيع الصمام الأورطي بالبالون: فهم تأثيرها على ديناميكيات الأعمال
يشهد سوق رأب الصمام الأورطي بالبالون نموًا سريعًا، مدفوعًا بالطلب المتزايد من المستخدم النهائي بسبب عوامل مثل تفضيلات المستهلكين المتطورة والتقدم التكنولوجي والوعي المتزايد بفوائد المنتج. ومع ارتفاع الطلب، تعمل الشركات على توسيع عروضها والابتكار لتلبية احتياجات المستهلكين والاستفادة من الاتجاهات الناشئة، مما يؤدي إلى زيادة نمو السوق.
تشير كثافة اللاعبين في السوق إلى توزيع الشركات أو المؤسسات العاملة في سوق أو صناعة معينة. وهي تشير إلى عدد المنافسين (اللاعبين في السوق) الموجودين في مساحة سوق معينة نسبة إلى حجمها أو قيمتها السوقية الإجمالية.
الشركات الرئيسية العاملة في سوق جراحة صمام الأبهر بالبالون هي:
- ب براون اس اي
- شركة تي تي الطبية
- بالتون
- بيكتون ديكنسون وشركاه
- شركة إدواردز للعلوم الحياتية
- بالت
إخلاء المسؤولية : الشركات المذكورة أعلاه ليست مرتبة بأي ترتيب معين.
- احصل على نظرة عامة على أهم اللاعبين الرئيسيين في سوق جراحة رأب الصمام الأورطي بالبالون
أخبار السوق والتطورات الأخيرة في مجال جراحة توسيع الصمام الأورطي بالبالون
يتم تقييم سوق رأب الصمام الأورطي بالبالون من خلال جمع البيانات النوعية والكمية بعد البحث الأولي والثانوي، والتي تتضمن منشورات الشركات المهمة وبيانات الجمعيات وقواعد البيانات. فيما يلي بعض التطورات في سوق رأب الصمام الأورطي بالبالون:
- أطلقت شركة أبوت أحدث جيل من نظام TAVI، Navitor، في الهند، مما يجعل الجهاز الأقل توغلاً متاحًا للأشخاص الذين يعانون من تضيق الأبهر الشديد والذين هم معرضون لخطر جراحي كبير أو شديد في البلاد. يتميز Navitor بسوار قماشي فريد من نوعه (NaviSeal) يعمل مع الدورة القلبية لتقليل أو القضاء على تدفق الدم عبر إطار الصمام، والمعروف باسم تسرب الصمام. (المصدر: شركة أبوت، بيان صحفي، ديسمبر 2022)
- أبرمت شركة Keystone Heart, Ltd. اتفاقية توزيع حصرية مع شركة InterValve Medical Inc. لبيع وتسويق محفظتها من منتجات رأب الصمام الأورطي بالبالون في الولايات المتحدة. وبموجب الاتفاقية، بدأت شركة Keystone Heart Ltd. في بيع قسطرات رأب الصمام الأورطي بالبالون V8 وTAV8 (InterValve Medical) في السوق الأمريكية. (المصدر: Keystone Heart, Ltd؛ بيان صحفي، مارس 2021)
تقرير سوق جراحة رأب الصمام الأورطي بالبالون والتغطية والنتائج المتوقعة
يقدم تقرير "حجم سوق جراحة صمام الأبهر بالبالون والتوقعات (2021-2031)" تحليلاً مفصلاً للسوق يغطي المجالات التالية:
- حجم سوق رأب الصمام الأورطي بالبالون وتوقعاته على المستويات العالمية والإقليمية والوطنية لجميع قطاعات السوق الرئيسية التي يغطيها النطاق
- اتجاهات سوق جراحة رأب الصمام الأورطي بالبالون بالإضافة إلى ديناميكيات السوق مثل العوامل المحركة والمقيدات والفرص الرئيسية
- تحليل مفصل لـ PEST و SWOT
- تحليل سوق جراحة رأب الصمام الأورطي بالبالون يغطي اتجاهات السوق الرئيسية والإطار العالمي والإقليمي والجهات الفاعلة الرئيسية واللوائح والتطورات الأخيرة في السوق
- تحليل المشهد الصناعي والمنافسة الذي يغطي تركيز السوق، وتحليل خريطة الحرارة، واللاعبين البارزين، والتطورات الأخيرة لسوق رأب الصمام الأورطي بالبالون
- ملفات تعريف الشركة التفصيلية
- التحليل التاريخي (سنتان)، سنة الأساس، التوقعات (7 سنوات) مع معدل النمو السنوي المركب
- تحليل PEST و SWOT
- حجم السوق والقيمة / الحجم - عالمي، إقليمي، بلد
- الصناعة والمنافسة
- مجموعة بيانات إكسل
التقارير الحديثة
شهادات العملاء
سبب الشراء
- اتخاذ قرارات مدروسة
- فهم ديناميكيات السوق
- تحليل المنافسة
- رؤى العملاء
- توقعات السوق
- تخفيف المخاطر
- التخطيط الاستراتيجي
- مبررات الاستثمار
- تحديد الأسواق الناشئة
- تحسين استراتيجيات التسويق
- تعزيز الكفاءة التشغيلية
- مواكبة التوجهات التنظيمية






















 احصل على عينة مجانية ل - سوق جراحة رأب الصمام الأورطي بالبالون
احصل على عينة مجانية ل - سوق جراحة رأب الصمام الأورطي بالبالون