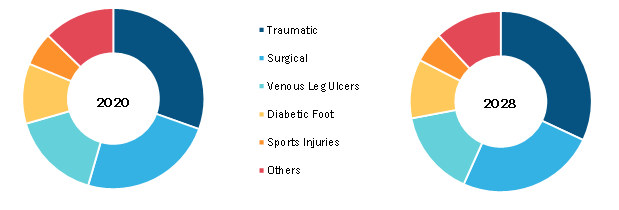
Negative pressure wound therapy (NPWT) is used for managing wide range of wound types such as traumatic, hard-to-heal, chronic wounds. It is also used to manage wounds that are covered with flaps or a skin graft. This therapy is transformed into a medical device such as a single-use NPWT device, and it is a small pocket-sized battery-operated device. As the name suggests, these are disposable and portable devices generally used for outpatient care.
The most notable market participants are Cardinal Health Inc; ConvaTec Group Plc; Carilex Medical; Genadyne Biotechnologies, Inc.; H and R Healthcare; Lohmann & Rauscher GmbH & Co. KG; Medela AG; Mölnlycke Health Care AB; Paul Hartmann Ag, and Smith & Nephew plc among others, occupying a considerable share of the market owing to their offerings to the market.
Smith & Nephew plc, and ConvaTec Group Plc – Notable Market Participants in Single-Use Negative Pressure Wound Therapy Devices Market
Market players are involved in offering the services that contribute to quality enhancement for services and cost efficiency within healthcare services. For instance, in September 2019, Smith+Nephew launched PICO 7Y in the US. It is a Single-Use Negative Pressure Wound Therapy System; based on Airlock Technology. Its sNPWT pump includes an innovative integrated Y connector that enables two dressings concurrently from one pump. Thus, permitting to address two wounds or incisions simultaneously. It has applications in different types of wounds such as closed surgical incisions, chronic and acute wounds
Prominent players in single-use negative pressure wound therapy devices market are focusing on majorly on inorganic strategies such as acquisitions and others to garner their significance and remain competitive in the market. Few of the important key developments from the industry are mentioned below:
|
Year |
News |
|
Mar-20 |
Smith & Nephew launched its new PICO 14 Single Use Negative Pressure Wound Therapy System. It has a pump duration of up to 14 days. The pump based on previous PICO sNPWT variants’ innovations and advantages. The pump is so enhanced that it requires less user intervention. |
|
Oct-18 |
ConvaTec Inc., received product approval from the US FDA for its Avelle Negative Pressure Wound Therapy system. The product I combined with the hydrofiber technology to treat chronic wounds at a speedy pace. |
|
May-17 |
Lohmann & Rauscher GmbH & Co. KG expanded its operations in North Africa, the company is partnership and third-party distributers has established its related business operation in the region. |
|
Jan-16 |
Medela AG as a part of its independent NPWT strategy launched its new dressing kit. previously the company had to source the NPWT dressing kits from Mölnlycke Health Care for the U.S. and Canadian markets. Thus, made independent of the in terms of the product supplies and business. |