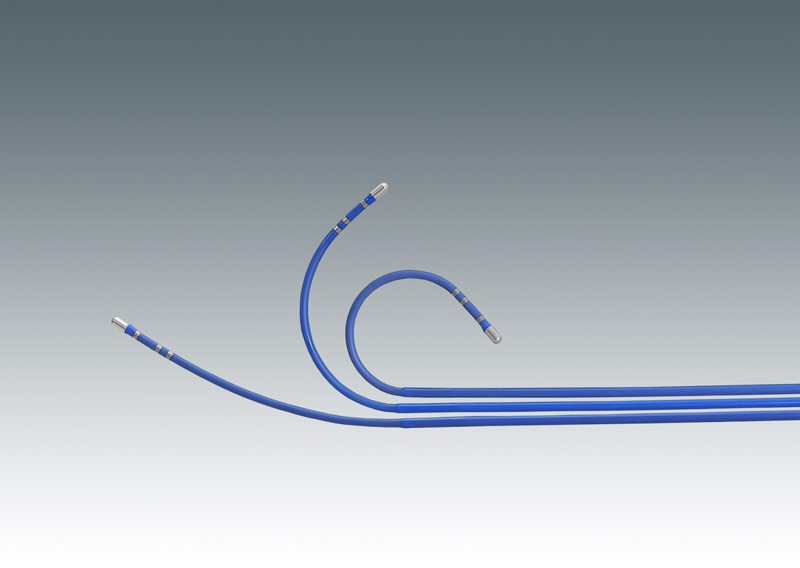
Electrophysiology catheters are designed to be used for better compatibility to perform standard electrophysiology studies. The catheter comprises of compatible stimulators/amplifiers that are purchased separately by the operator. Depending on the features of the simulator/amplifier, pacing, and recording protocols can be performed inside the human’s body to determine abnormalities. For example, electrophysiology catheters are performed inside the heart (intracardiac) or through the esophagus (transesophageal) for determining electrical properties of the atrium and ventricle. High prevalence of cardiovascular disorders and technological advancement are expected to boost the market growth
Abbott and Johnson and Johnson Services, Inc. – Notable Market Players in Electrophysiology catheter Market
Market leaders operating in the market have undertaken various organic growth strategies in the electrophysiology catheter market. The electrophysiology catheter market majorly consists of the players St. Jude Medical (Abbott), Boston Scientific Corporation, Stryker Corporation, Medtronic, APT MEDICAL INC., MicroPort Scientific Corporation, Biosense Webster (Johnson and Johnson Services, Inc.), Biotronik, Inc. The companies have been implementing various strategies that have helped the growth of the company and in turn have brought about various changes in the market. The companies have utilized organic strategies such as launches, expansion, and product approvals. Moreover, the companies have utilized inorganic strategies including mergers & acquisitions, partnership, and collaboration.
Below is the list of the growth strategies done by the players operating in the electrophysiology catheter market:
| Company | Month & Year | Category | Description |
| MicroPort Scientific Corporation | Mar-2021 | Product Approval | MicroPort obtained registration approval from the Australia Therapeutic Goods Administration (TGA) for the medical devices and accessories, including Columbus 3D EP Navigation System, Cardiac Electrophysiology Stimulator, OptimAblate Cardiac RF Generator, among others. For surgeons, the Columbus 3D EP Navigation System added safety and efficacy in their procedures as it captured and analyzed the electrophysiological activity of the heart and displayed a real-time 3D heart image. |
| Abbott | Nov-2020 | Product Launch | Abbott launched the new EnSite X EP System and received CE Mark and approval throughout Europe and Australia. The EnSite X System offered the option to navigate the cardiac anatomy two different ways on one platform and expanded the company’s electrophysiology portfolio. Cardiac mapping systems allowed electrophysiologists to create a heart map and helped them get a clear picture of the electrical signals that control cardiac rhythms. |
| Johnson and Johnson Services, Inc. | Oct-2020 | Product Approval | Johnson & Johnson Medical Devices Companies and Biosense Webster’s ablation catheter was approved by the FDA in order to treat patients with persistent atrial fibrillation. The catheter approval was based on findings from the PRECEPT study. The study found that ablation with a porous tip contact force-sensing catheter was safe and effective in patients with persistent AF. |
| Boston Scientific Corporation | Oct-2021 | Mergers and Acquisitions | Boston Scientific Corporation entered into a definitive agreement to acquire Baylis Medical Company Inc. for an upfront payment of $1.75 billion, subject to closing adjustments. The acquisition expanded the company’s electrophysiology and structural heart product portfolios to include the radiofrequency (RF) NRG and VersaCross Transseptal Platforms and a family of guidewires, sheaths and dilators used to support left heart access. |
| Medtronic | May-2019 | Collaboration | Medtronic collaborated with Philips to advance the image-guided treatment of paroxysmal atrial fibrillation (PAF), a common heart rhythm disorder. Through the agreement, Medtronic facilitated products sales on behalf of Philips to provide an innovative, integrated image guidance solution to enable electrophysiologists to better perform cryoablation procedures. |