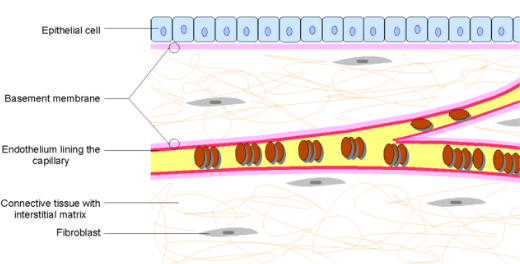
The irritable bowel syndrome (IBS) diagnostics market is highly competitive in nature with considerable number of players, having a high level of consolidation overall revenue share. Most of the companies operating in the irritable bowel syndrome (IBS) diagnostics market are present globally and have wide distribution and sales network through partnerships or authorized dealers.
The most notable market participants are Commonwealth Diagnostics International, Genova Diagnostics, Gemelli Biotech, Prometheus Laboratories, Inova Diagnostics, Inc., Biohit Oyj, Aerodiagnostics, LLC., Metabolic Solutions, Inc., Sysmex Corporation, BÜHLMANN Laboratories AG occupying a considerable share of the market owing to their offerings to the market.
Biohit Oyj, Sysmex Europe GmbH, Gemelli Biotech, Commonwealth Diagnostics International, Inc – Notable Market Participants in Irritable Bowel Syndrome (IBS) Diagnostics Industry
Market leaders are involved in extensive research for the development of new irritable bowel syndrome (IBS) diagnostics with better efficiency and treatment outcomes. For instance, in February 2021, The American Medical Association (AMA) has granted Commonwealth Diagnostics International, Inc.’s IBSchek a PLA code; the company will now accept insurance, allowing more patients at-home access. Commonwealth Diagnostics International, Inc. (CDI) will begin accepting insurance immediately for their proprietary IBSchek Capillary Collection Kit. Clinically validated in 2015 and revalidated internationally, IBSchek is the most reliable diagnostic blood test for both diarrhea-predominant and mixed-symptom IBS (IBS-D/M) in the market.
Many well-known as well as small local companies are present in the market to provide diversified products to its customers. The larger firms are adopting the strategy of acquiring small firms to enhance its product portfolio and expand its footprint in different geographies. Additionally various companies are also undergoing other strategic alliances such as collaborations and others to garner their significance and remain competitive in the market. Few on the important key developments from the industry are mentioned below:
|
Year |
News |
Region |
|
2019 |
BÜHLMANN Laboratories AG received United States Food and Drug Administration (FDA) 510(k) clearance of its BÜHLMANN fCAL turbo. This turbidimetric test was successfully launched all over the world and is now available on the U.S. market. The BÜHLMANN fCAL turbo is an in vitro diagnostic assay intended for the quantitative measurement of fecal calprotectin (fCAL), a neutrophilic protein that is a marker of intestinal mucosal inflammation, in human stool. The BÜHLMANN fCAL turbo aids in the diagnosis of inflammatory bowel disease (IBD), specifically Crohn’s disease (CD) and ulcerative colitis (UC) and aids in the differentiation of IBD from irritable bowel syndrome (IBS) in conjunction with other laboratory and clinical findings. |
North America |
|
2019 |
Gemelli Biotech announced a partnership with Kuwait-based Advanced Medical German Co. (AMG), one of the leading international distributors of medical products and services in the Middle East. Through the partnership, AMG will distribute Gemelli’s proprietary diagnostic blood test for IBS, ibs-smart, in major territories in the Middle East and North Africa, including Kuwait, United Arab Emirates, Qatar, Saudi Arabia, Egypt and Turkey, among others. |
Middle East & Africa |
|
2018 |
Sysmex Europe GmbH and Sentinel Diagnostics will continue their partnership, which gives Sysmex Europe the distribution rights for Sentinel’s newest FIT line assay: CALiaGold. Sysmex Europe and Sentinel agreed to continue and expand their partnership, which now includes the new CALiaGold in combination with FOB Gold and SENTiFIT 270 Analyser. |
Europe |