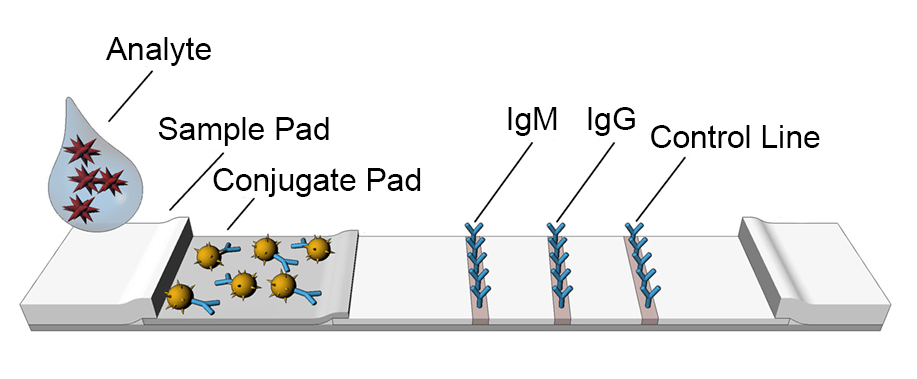
Lateral flow assay (LFA), known as lateral flow immunochromatographic assay, is a simple paper-based device intended for detecting the presence or absence of a target analyte in a liquid sample (matrix) without the need for specialized or costly equipment. The lateral flow assay is used for medical diagnostics, home testing, point-of-care testing, and laboratory use. The lateral flow assay market is presumed to account for the highest CAGR from 2023 to 2028. The growth is attributable to the growing usage of home-based lateral flow assay devices and growing demand for point-of-care testing.
Abbott Laboratories and Danaher Corp – Notable Market Players in Lateral Flow Assay Market
Key factors that are driving the growth of the lateral flow assay market are the increasing use of home-based assay kits and rising popularity of point-of-care testing drives the growth of the lateral flow assays market. However, the market is likely to get impacted by the inconsistent assay results due to procedural limitations during the forecast period.
The lateral flow assay market is majorly comprised of top players involving F. Hoffmann-La Roche Ltd, Thermo Fisher Scientific Inc, Abbott Laboratories, Merck KGaA; Hologic Inc; Qiagen NV; bioMerieux SA; Bio-Rad Laboratories Inc; Access Bio Inc; Danaher Corp; among others.
The companies listed above are implementing various strategies such as product launches, acquisitions, mergers, and partnerships, which have resulted in the company’s growth and, in turn, have brought about various changes in the worldwide market. Additionally, the companies have adopted several inorganic and organic strategies for accelerating their growth and improving their market position.
Below is the list of the growth strategies done by the players operating in the lateral flow assay market:
|
Year |
News |
|
Mar-2023 |
Roche announced that the FDA approved the Ventana PD-L1 (SP263) assay |
|
Dec-2022 |
Roche announced FDA 510(k) clearance of its Elecsys Beta-Amyloid (1-42) CSF II (Abeta42) |
|
Jun-2021 |
Thermo Fisher Scientific launched its CE-IVD-marked TaqPath COVID-19 Fast PCR Combo Kit 2.0. |
|
May-2022 |
Abbott received FDA clearance for its Alinity m STI assay. The test simultaneously detects and differentiates |
|
Ded-2021 |
Merck announced that its Life Science business sector for the construction of a |
|
Jul-2021 |
Hologic obtained a CE Mark for the use of saliva samples with the |