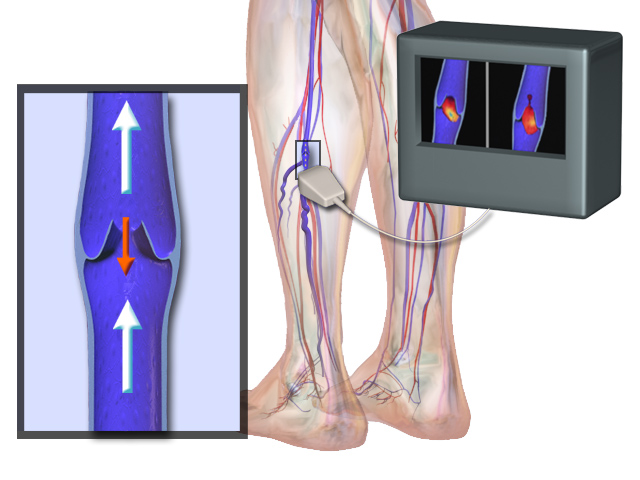
Nitinol refers to an alloy of nickel and titanium that is rapidly becoming a metal of choice for composition of various medical devices in the healthcare industry. Nitinol widely finds its applications as self-expanding grafts, baskets, filters, graft-supporting systems, and others. Nitinol alloys are most known for their super-elasticity and thermal shape memory.
A few of the most notable companies occupying a considerable share of the market are Cook Medical LLC; Abbott Laboratories; Zimmer Biomet Holdings, Inc; B. Braun Melsungen AG; Boston Scientific Corporation; Becton, Dickinson and Company (BD); Arthrex, Inc.; W. L. Gore & Associates, Inc; Terumo Corporation, and Nordson Corporation.
Boston Scientific Corporation and. Becton, Dickinson and Company. – Notable Participants in Nitinol Medical Devices Market
Market leaders are involved in partnerships, product launches, acquisitions, and other strategies to improve their performance and consolidate the market position. For instance, in February 2021 Cook Medical has announced that the Approach Authority Workhorse Microwire Guide is now commercially available in the United States and Canada. It combines resilient nitinol tip technology with a hydrophilic coating. These wire guides can be used for various vascular procedure.
Prominent players in the Nitinol medical devices market are focusing on organic strategies such as product launches, product approvals, and geographic and manufacturing expansions. These strategies help them strengthen their market positions, along with allowing them to broaden their geographic footprints. Additionally, various companies are undertaking acquisitions to garner their significance and remain competitive in the market. A few of the vital developments in the nitinol medical devices market are mentioned below:
|
Year |
News |
Region |
|
2020 |
Terumo has received Breakthrough Device Designation from US Food and Drug Administration (FDA) for Thoraflex Hybrid. Thoraflex Hybrid is a single use medical device combining a gelatin-sealed woven polyester graft with a Nitinol self-expanding stent graft and is indicated for the surgical repair or replacement of damaged or diseased vessels of the aortic arch and descending aorta. |
America |
|
2019 |
BD (Becton, Dickinson and Company) has received U.S. Food and Drug Administration premarket approval for the Venovo venous stent. The Venovo venous stent is a flexible nitinol stent specifically designed to reopen blocked iliac and femoral veins in order to maintain adequate blood flow. |
America |