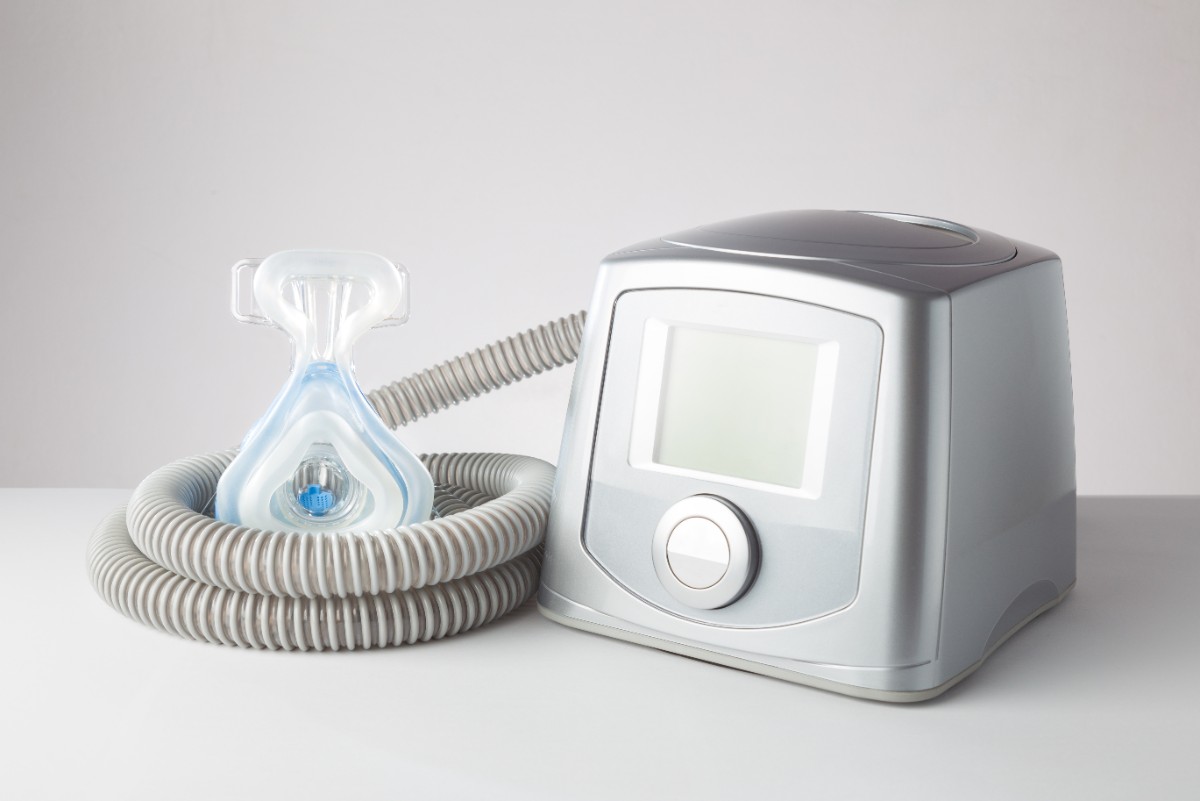
Respiratory care devices are those medical instruments that are used for the diagnosis, monitoring, and care of patients who are suffering from various respiratory diseases. Major devices such as oxygen concentrator, ventilators, inhalers, nebulizers, spirometers, positive airway pressure (PAP) are primarily used for respiratory disease’s diagnostic, therapeutic, and monitoring. Introduction of innovative product launches and high adoption of cutting edge technology and integration of digital and sensor based technologies for creating a smart, portfolio, and connected respiratory care devices will further aid the growth of the market.
Koninklijke Philips N.V. & ResMed Inc.–are Notable Market Players in Respiratory Care Devices Market
The growth of the market is attributed to the increasing prevalence of infectious respiratory diseases, rapidly increasing cases of asthma and COPD, rising number of product launches and approvals, and growing R & D investment for respiratory care devices. However, cutthroat competition among market players and unfavorable reimbursement scenarios hampers the respiratory care devices market growth.
Market leaders operating in the market have undertaken various organic and inorganic growth strategies. The generic injectables market majorly consists of players like Medtronic, Masimo, Thermo Fisher Scientific Inc., Dragerwerk AG & Co. KGaA, Invacare Corporation, Getinge AB, Nihon Kohden Corporation, Air Liquide, and Teleflex Incorporated. Koninklijke Philips N.V. and ResMed Inc., are the topmost two companies in the market.
Several in organic approaches, such as product launches, and expansion in the respiratory care devices, have resulted in the growth of the market. Likewise, inorganic strategies such as mergers & acquisitions, and collaboration have helped the company to strengthen its revenue, which allows the company to hold a strong position in the market.
Below is the list of the growth strategies done by the players operating in the respiratory care devices:
| Year | News |
| Sep-2021 | To address potential health hazards posed by the machines, Philips began repairing and replacing millions of breathing devices in the United States and most of its other regions this month. |
| Jun-2021 | Royal Philips recalled breathing devices and ventilators after it found possible health risks linked to the polyester-based polyurethane (PE-PUR) sound abatement foam used in these products. The recalled products are Bi-Level Positive Airway Pressure (Bi-Level PAP), Continuous Positive Airway Pressure (CPAP) and mechanical ventilator devices. |
| Nov-2020 | Philips has introduced new non-invasive ventilator for COPD patients. The Ventilator BiPAP A40 EFL, which is claimed to be the first and only non-invasive ventilator that enables health care professionals to automatically screen and detect expiratory flow limitation (EFL) to deliver optimal homecare therapy. |
| Apr-2020 | Royal Philips launched its new Philips Respironics E30 ventilator, a versatile and easy-to-use noninvasive and invasive ventilator designed for large-scale production. Philips received an emergency use authorization (EUA) for the E30 from the FDA on April 8, 2020, the company said in a statement. |
| Feb-2019 | Royal Philips has announced a ‘No-cost EMI’ 1 offer on its sleep and respiratory solutions from Respironics. Patients with Chronic Obstructive Pulmonary Disease (COPD), a lung disease associated with long breathing difficulties, and Obstructive Sleep Apnea (OSA), a chronic condition characterized by interrupted breathing during sleep, will benefit from these remedies. |
| Apr-2021 | ResMed has expanded AirView for Ventilation, its cloud-based remote monitoring and management platform for respiratory care patients, in India |
| Nov-2019 | ResMed announced an upgraded AirView for Respiratory program that includes management by exception, providing HMEs and physicians with quicker access to essential respiratory patient data and allowing them to provide better quality care |
| Jan-2019 | ResMed, a leader in respiratory care medical devices and out-of-hospital care software, announced that its premier portable oxygen concentrator, Mobi, is now widely available in the United States |