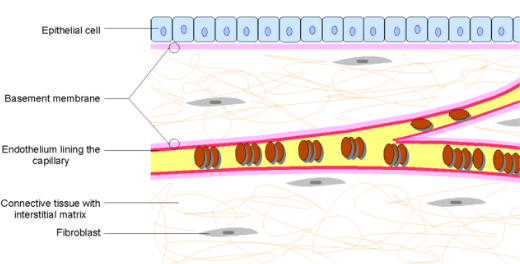
High incidence of sepsis due to increasing nosocomial infections and rise in demand for rapid diagnostic tests leading to increasing product launches are the major factors driving the sepsis diagnostics market. Sepsis is a severe medical condition in which the body responds to an infection. It is a life-threatening medical emergency caused due to contamination with bacteria, viruses, or fungi. The sepsis diagnostics market is majorly comprised of top players involving Abbott, F. HOFFMANN-LA ROCHE LTD., Immunexpress Inc., BD, Danaher, Luminex Corporation, THERMO FISHER SCIENTIFIC INC., bioMerieux SA, T2 Biosystems, Inc., and Axis-Shield Diagnostics Ltd.
BioMerieux and Abbott- Notable Market Players in Sepsis Diagnostics Market
The companies listed above are implementing various strategies that have resulted in the growth of the company and in turn, have brought about various changes in the worldwide market. Additionally, the companies have adopted several inorganic and organic strategies for accelerating their growth and improving their market position.
Below is the list of the growth strategies done by the players operating in the sepsis diagnostics market:
| Year | News |
| Apr-2022 | BioMérieux entered into an agreement to acquire Specific Diagnostics, a privately held U.S. based company that has developed a rapid antimicrobial susceptibility test (AST) system. With the addition of SPECIFIC REVEAL Rapid AST, the unique and comprehensive bioMérieux Sepsis Solution allows same-day AST results for Gram-negative bacteria to enable more targeted therapy and improve patient outcomes. SPECIFIC REVEAL seamlessly integrates with bioMérieux’s Sepsis Solution including BACT/ALERT VIRTUO, BIOFIRE BCID2, VIDAS PCT and VITEK 2. |
| Mar-2022 | T2 Biosystems, Inc. announced that the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the U.S. Department of Health and Human Services, provided an additional US$4.4 million in funding for the multiple-year cost-share contract between BARDA and T2 Biosystems. The additional funding is used to advance the U.S. clinical trials for the T2Biothreat Panel and T2Resistance Panel, and to advance the development of the Company’s comprehensive panel for the detection of bloodstream infections and antimicrobial resistance, and next-generation instrument. |