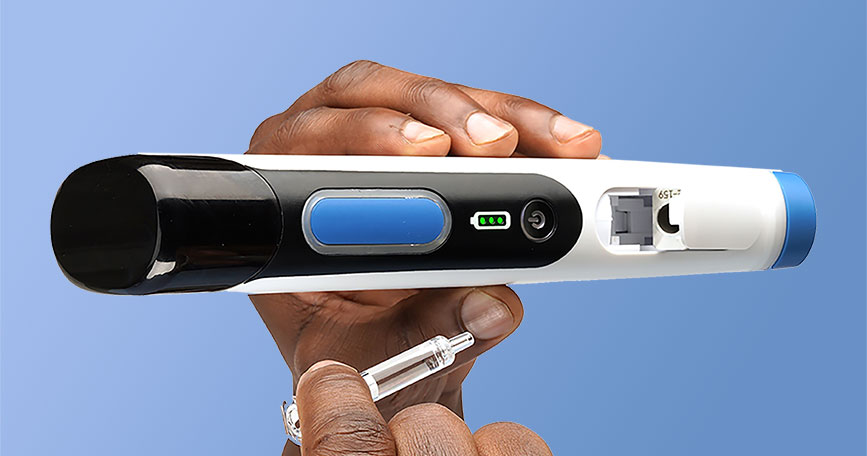
Needle free injectors are designed to deliver the drugs subcutaneously, intramuscularly, and intradermally by using forces such as, Lorentz force, shock waves, pressure by gas or electrophoresis which propels the drug through the skin. Fillable needle free injectors are standard and sustainable alternative to syringes. Fillable needle free injector has few advantages over prefilled injectors such as limited chances of extractables and leaching (E&L) that reduces chances of drug interaction. These E&L can interfere with the drug molecules and could compromise the effectiveness of a drug. For instance, silicone (used in prefilled syringe) can be a source of E&L or particles, which could alter sensitive proteins under certain conditions—causing them to aggregate or change their form. Prefilled needle free injectors are used to package injectable drugs and vaccines. Prefilled syringes may contain drugs such as antithrombotic agents, vaccines, blood stimulants, interferons, and rheumatoid arthritis medications. Due to the rapid adoption of prefilled needle free injection, these injections have emerged as fastest growing choice for unit dose drugs.
INOVIO Pharmaceuticals and Zealand Pharma A/S – Notable Market Players in Needle Free Injection System Market
The needle free injection system market majorly consists of the players such as Crossject; INOVIO Pharmaceuticals; Zealand Pharma A/S; PharmaJet; Portal Instruments; Medical International Technologies (MIT Canada) Inc.; NuGen Medical Devices; Ferring B.V.; National Medical Products, Inc. and Aijex Pharma International Inc. These growth strategies have aided the market players in the expansion of their business and enhanced their geographic presence. Additionally, growth strategies such as acquisitions and partnerships helped in strengthening their customer base and increasing the product portfolio. The companies have utilized strategies such as product launch, product approval, expansion of their product portfolio, and area expansion for the growth of their organizations. In addition, the companies have invested their efforts to develop products and have received approvals from regulatory bodies such as US FDA for their products.
Below is the list of the growth strategies done by the players operating in the Asparaginase market:
| Year | News |
| Jun-2022 | NuGen Medical Devices Inc., a leader in needle-free drug delivery, has announced that it will be launching a needle-free injection device specifically designed for domesticated household pets. The PetJet will allow pet owners and veterinarians to safely and quickly inject various types of medication without the fear of hurting their pets with a traditional hypodermic needle. |
| Jun-2022 | BARDA places a firm order of US$60m to CROSSJECT for initial procurement of ZENEO Midazolam into the U.S. Strategic National Stockpile1 as soon as FDA Authorization is obtained |
| May-2022 | Gerresheimer AG is expanding its portfolio of highly-innovative platform technologies for drug delivery. Gerresheimer, a leading provider of healthcare & beauty solutions and drug delivery systems for pharma, biotech and cosmetics, announced an investment into US-based Portal Instruments, a developer of a next-generation needle-free drug delivery technology. Together, the partners aim at transforming the administration of injectable medicines and improving the patient’s experience, especially for those with chronic diseases. |
| May-2021 | Crossject and Cenexi are strengthening their partnership to ensure the ramp-up of industrial production of the ZENEO needle-free auto-injector. With this agreement, Crossject renews its confidence in Cenexi for this new strategic phase. |