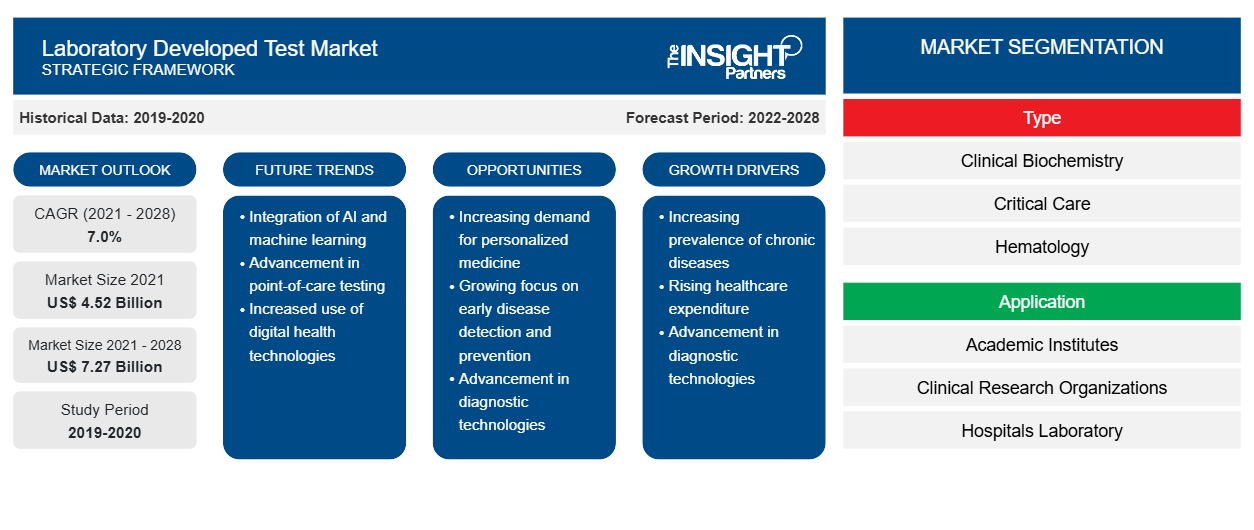
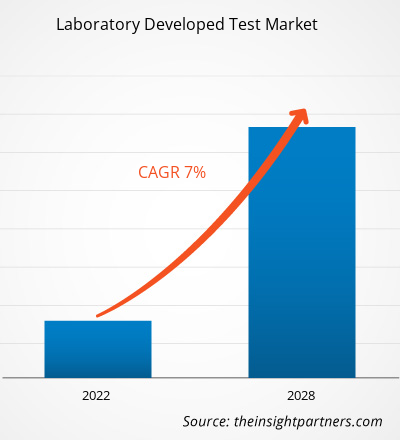
Si prevede che il mercato dei test sviluppati in laboratorio raggiungerà i 7.269,3 milioni di dollari entro il 2028, rispetto ai 4.524,75 milioni di dollari del 2021; si prevede che crescerà a un CAGR del 7,0% nel periodo 2021-2028.
Un test sviluppato in laboratorio (LDT) è un tipo di test diagnostico in vitro progettato e utilizzato all'interno di un singolo laboratorio. Questi test possono essere utilizzati per stimare o distinguere analiti quali proteine, biomolecole/composti (glucosio, colesterolo, ecc.) e DNA estratto da campioni raccolti da soggetti umani. L'espansione dei metodi di diagnostica in vitro automatizzata (IVD) per laboratori e dispensari per rendere analisi precise e prive di errori sta alimentando la crescita del mercato dei test sviluppati in laboratorio.
La crescita del mercato dei test sviluppati in laboratorio è attribuita principalmente a fattori quali l'aumento di casi di cancro e disturbi genetici e il gran numero di lanci di prodotti. Tuttavia, il cambiamento del panorama normativo sta ostacolando la crescita del mercato. Ad esempio, in Europa, la conformità al Regolamento sui dispositivi in vitro (IVDR) sarà obbligatoria per tutti i test diagnostici in vitro da maggio 2022; il regolamento mira a garantire l'efficacia clinica e la sicurezza dei test medici, trasformando così il settore diagnostico, il che è una grande preoccupazione per gli operatori del mercato.
Personalizza questo report in base alle tue esigenze
Riceverai la personalizzazione gratuita di qualsiasi report, comprese parti di questo report, o analisi a livello nazionale, pacchetto dati Excel, oltre a usufruire di grandi offerte e sconti per start-up e università
- Scopri le principali tendenze di mercato in questo rapporto.Questo campione GRATUITO includerà analisi di dati che spaziano dalle tendenze di mercato alle stime e alle previsioni.
Approfondimenti di mercato
La ricerca continua sui medicinali personalizzati offre opportunità di crescita agli operatori del mercato dei test sviluppati in laboratorio
Gli LDT svolgono un ruolo fondamentale nello sviluppo di medicinali personalizzati che probabilmente si riveleranno mezzi promettenti per affrontare le malattie attraverso trattamenti o cure efficaci finora elusi. Secondo la Personalized Medicine Coalition, i medicinali personalizzati rappresentavano solo il 5% delle nuove entità molecolari approvate dalla FDA nel 2005; tuttavia, nel 2016, questo numero è salito a oltre il 25%. Inoltre, il 42% di tutti i composti e il 73% dei composti oncologici in fase di sviluppo hanno il potenziale per fungere da medicinali personalizzati. Le aziende biofarmaceutiche hanno quasi raddoppiato i loro investimenti in R&S in farmaci personalizzati negli ultimi cinque anni e si prevede che aumenteranno ulteriormente i loro investimenti del 33% nei prossimi cinque anni. I ricercatori biofarmaceutici prevedono anche un aumento del 69% nello sviluppo di medicinali personalizzati nei prossimi cinque anni. I test di laboratorio vengono utilizzati per diagnosticare le malattie e prevedere e monitorare la risposta ai farmaci, nonché per ottenere dati informatici necessari per algoritmi predittivi complessi. play a vital role in the development of personalized medicines that are likely to prove as promising means of tackling diseases through far eluded effective treatments or cures. As per the Personalized Medicine Coalition, personalized medicines accounted for only 5% of the new FDA-approved molecular entities in 2005; however, in 2016, this number rose to more than 25%. Additionally, 42% of all compounds and 73% of oncology compounds in the pipeline have the potential to serve as personalized medicines. Biopharmaceutical companies have nearly doubled their R&D investments in personalized drugs in the last five years, and they are further expected to increase their investments by 33% in the next five years. Biopharmaceutical researchers also predict a 69% increase in the development of personalized medicines in the next five years. Laboratory tests are used to diagnose illness and predict and monitor drug response as well as to obtain informatics data needed for complex predictive algorithms.
I farmaci personalizzati stanno diventando il marchio di fabbrica del trattamento del cancro; si tratta di un approccio in continua evoluzione che si basa sulla personalizzazione dei trattamenti in base al patrimonio genetico individuale. Nel 2019, la FDA ha approvato 12 farmaci personalizzati per indagare e affrontare le cause profonde della malattia, combinando così la medicina di precisione nell'assistenza clinica. La crescente domanda di medicina personalizzata offre significative opportunità di crescita per la crescita degli operatori che operano nel mercato dei test sviluppati in laboratorio.
Informazioni basate sul tipo
Il mercato dei test sviluppati in laboratorio, per tipo, è segmentato in biochimica clinica, terapia intensiva, ematologia, microbiologia, diagnostica molecolare, immunologia e altri. Si prevede che il segmento della diagnostica molecolare detenga la quota maggiore del mercato nel 2021. Tuttavia, si prevede che il segmento dell'ematologia registri il CAGR più elevato nel mercato durante il periodo di previsione.CAGR in the market during the forecast period.
Approfondimenti basati sulle applicazioni
Per applicazione, il laboratorio ha sviluppato applicazioni di mercato di prova in istituti accademici, organizzazioni di ricerca clinica, laboratori ospedalieri, centri diagnostici specialistici e altri. Si stima che il segmento dei laboratori ospedalieri rappresenti la quota di mercato maggiore nel 2021. Tuttavia, si prevede che il segmento dei centri diagnostici specialistici registrerà il CAGR più elevato nel mercato durante il periodo di previsione.CAGR in the market during the forecast period.
I lanci e le approvazioni di prodotti sono strategie comunemente adottate dalle aziende per espandere la loro presenza globale e i loro portafogli di prodotti. Inoltre, i test di mercato sviluppati in laboratorio si concentrano sulla strategia di partnership per ampliare la loro clientela, il che, a sua volta, consente loro di mantenere il loro marchio a livello globale.
Il rapporto suddivide il mercato dei test sviluppati in laboratorio come segue
In base al tipo, il mercato dei test sviluppati in laboratorio è segmentato in biochimica clinica, terapia intensiva, ematologia, microbiologia, diagnostica molecolare, immunologia e altri. In base all'applicazione, il mercato dei test sviluppati in laboratorio è segmentato in istituti accademici, organizzazioni di ricerca clinica, laboratori ospedalieri, centri diagnostici specialistici e altri. In base alla geografia, il mercato dei test sviluppati in laboratorio è segmentato in Nord America (Stati Uniti, Canada e Messico), Europa (Regno Unito, Germania, Francia, Italia, Spagna e resto d'Europa), Asia Pacifico (Cina, Giappone, India, Australia, Corea del Sud e resto dell'Asia Pacifico), Medio Oriente e Africa (EAU, Arabia Saudita, Sud Africa e resto del Medio Oriente e Africa) e America meridionale e centrale (Brasile, Argentina e resto dell'America meridionale e centrale).
Approfondimenti regionali sul mercato dei test sviluppati in laboratorio
Le tendenze regionali e i fattori che influenzano il Laboratory Developed Test Market durante il periodo di previsione sono stati ampiamente spiegati dagli analisti di Insight Partners. Questa sezione discute anche i segmenti e la geografia del Laboratory Developed Test Market in Nord America, Europa, Asia Pacifico, Medio Oriente e Africa e Sud e Centro America.
- Ottieni i dati specifici regionali per il mercato dei test sviluppati in laboratorio
Ambito del rapporto di mercato sui test sviluppati in laboratorio
| Attributo del report | Dettagli |
|---|---|
| Dimensioni del mercato nel 2021 | 4,52 miliardi di dollari USA |
| Dimensioni del mercato entro il 2028 | 7,27 miliardi di dollari USA |
| CAGR globale (2021 - 2028) | 7,0% |
| Dati storici | 2019-2020 |
| Periodo di previsione | 2022-2028 |
| Segmenti coperti | Per tipo
|
| Regioni e Paesi coperti | America del Nord
|
| Leader di mercato e profili aziendali chiave |
|
Test di laboratorio sviluppato Mercato di attori Densità: comprendere il suo impatto sulle dinamiche aziendali
Il mercato dei test sviluppati in laboratorio sta crescendo rapidamente, spinto dalla crescente domanda degli utenti finali dovuta a fattori quali l'evoluzione delle preferenze dei consumatori, i progressi tecnologici e una maggiore consapevolezza dei vantaggi del prodotto. Con l'aumento della domanda, le aziende stanno ampliando le loro offerte, innovando per soddisfare le esigenze dei consumatori e capitalizzando sulle tendenze emergenti, il che alimenta ulteriormente la crescita del mercato.
La densità degli operatori di mercato si riferisce alla distribuzione di aziende o società che operano in un particolare mercato o settore. Indica quanti concorrenti (operatori di mercato) sono presenti in un dato spazio di mercato in relazione alle sue dimensioni o al valore di mercato totale.
Le principali aziende che operano nel mercato dei test sviluppati in laboratorio sono:
- Diagnostica di ricerca incorporata
- F. HOFFMANN-LA ROCHE LTD.,
- QIAGEN
- Illumina, Inc.,
- Eurofins Scientific
Disclaimer : le aziende elencate sopra non sono classificate secondo un ordine particolare.
- Ottieni la panoramica dei principali attori del mercato dei test sviluppati in laboratorio
Profili aziendali
- Diagnostica di ricerca incorporata
- F. HOFFMANN-LA ROCHE LTD.,
- QIAGEN
- Illumina, Inc.,
- Eurofins Scientific
- Biodesix
- Biotecnologie adattive
- Bioteranostica
- Rosetta Genomics Ltd.,
- Salute Guardante
- Analisi storica (2 anni), anno base, previsione (7 anni) con CAGR
- Analisi PEST e SWOT
- Valore/volume delle dimensioni del mercato - Globale, regionale, nazionale
- Industria e panorama competitivo
- Set di dati Excel

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends
Segment Covered
This text is related
to segments covered.

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America
Country Scope
This text is related
to country scope.
Domande frequenti
The laboratory developed test market is led by type molecular diagnostics segment is growing significantly due to the increasing attention to the development of genetic therapeutics. The growing research in human genomics is playing a vital role in developing laboratory developed tests. The technological advantages of molecular tests make them compelling diagnostic tools, and they have become valuable for detecting a wide range of genetic diseases. For instance, in November 2017, Edico Genome launched the DRAGEN Clinical Genomics Information System (CGIS).
The automation of LDTs can significantly boost productivity and simplify compliance procedures. Clinical laboratories are under huge pressure to manage increasing test volumes and conduct more complex diagnostic assays, which further underlines the need for flexible automation systems. Many MedTech companies are offering automation solutions to help streamline clinical laboratory processes. For instance, Roche Cobas Omni–Utility Channel is beneficial for both IVD assays and high-volume LDTs. It is designed to meet the growing needs of efficient workflows in laboratories, as it can assist in different stages ranging from sample processing to quick data interpretation. It can run up to 96 results in about 3 hours and up to 864 results in 8 hours, thereby boosting the efficiency of laboratories.
The growth of the region is attributed to factors such as rising public–private partnerships, and increasing funding activities are widely enhancing the performance of medical devices. Moreover, presence of well-developed healthcare infrastructure and government support are some of the prominent factors propelling the market growth in Asia Pacific. In Asia Pacific, India is the largest market for laboratory developed test. The market growth in Asia Pacific is mainly attributed to factors such as the growing usage of laboratory developed test in combating Covid-19, increasing incidents of cancer and genetic disorders, large number of product launches. However concerns high capital investment for setting up advanced labs hinders the market growth in Asia Pacific.
A laboratory developed test (LDT) is a type of in vitro diagnostic test that is designed and used within a single laboratory. These tests can be utilized to estimate or distinguish an extensive assortment of analytes materials such as proteins, chemical compounds like glucose or cholesterol, or DNA, from a specimen received from human anatomy. The expansion of automated in vitro diagnostics (IVD) methods for labs and dispensaries to render precise, and error-free analysis is anticipated to fuel the increment.
The growth of the laboratory developed test market is mainly attributed to factors such as the increasing incidents of cancer and genetic disorders, large number of product launches. However, changing regulatory landscape for instance, in Europe, the In-Vitro Device Regulation (IVDR) compliance will be mandatory for all in vitro diagnostic tests from May 2022; the regulation aims to secure the clinical effectiveness and safety of medical tests, thus transforming the diagnostic industry is a great concern to hinder the market growth. In August 2021, eMed, a telehealth company democratizing healthcare through digital-point-of-care solutions, and Quest Diagnostics the world's leading provider of diagnostic information services, are collaborated to bring clinician-guided rapid antigen testing for COVID-19 to employers seeking to foster safer environments by decreasing the risk of COVID-19 exposure in their workplaces which is contributing to the growth of the target market. Such strategic steps are also projected to drive the market growth.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Laboratory Developed Test Market
- Quest Diagnostics Incorporated
- F. HOFFMANN-LA ROCHE LTD.,
- QIAGEN
- Illumina, Inc.,
- Eurofins Scientific
- Biodesix
- Adaptive Biotechnologies
- Biotheranostics
- Rosetta Genomics Ltd.,
- Guardant Health
The Insight Partners performs research in 4 major stages: Data Collection & Secondary Research, Primary Research, Data Analysis and Data Triangulation & Final Review.
- Data Collection and Secondary Research:
As a market research and consulting firm operating from a decade, we have published and advised several client across the globe. First step for any study will start with an assessment of currently available data and insights from existing reports. Further, historical and current market information is collected from Investor Presentations, Annual Reports, SEC Filings, etc., and other information related to company’s performance and market positioning are gathered from Paid Databases (Factiva, Hoovers, and Reuters) and various other publications available in public domain.
Several associations trade associates, technical forums, institutes, societies and organization are accessed to gain technical as well as market related insights through their publications such as research papers, blogs and press releases related to the studies are referred to get cues about the market. Further, white papers, journals, magazines, and other news articles published in last 3 years are scrutinized and analyzed to understand the current market trends.
- Primary Research:
The primarily interview analysis comprise of data obtained from industry participants interview and answers to survey questions gathered by in-house primary team.
For primary research, interviews are conducted with industry experts/CEOs/Marketing Managers/VPs/Subject Matter Experts from both demand and supply side to get a 360-degree view of the market. The primary team conducts several interviews based on the complexity of the markets to understand the various market trends and dynamics which makes research more credible and precise.
A typical research interview fulfils the following functions:
- Provides first-hand information on the market size, market trends, growth trends, competitive landscape, and outlook
- Validates and strengthens in-house secondary research findings
- Develops the analysis team’s expertise and market understanding
Primary research involves email interactions and telephone interviews for each market, category, segment, and sub-segment across geographies. The participants who typically take part in such a process include, but are not limited to:
- Industry participants: VPs, business development managers, market intelligence managers and national sales managers
- Outside experts: Valuation experts, research analysts and key opinion leaders specializing in the electronics and semiconductor industry.
Below is the breakup of our primary respondents by company, designation, and region:
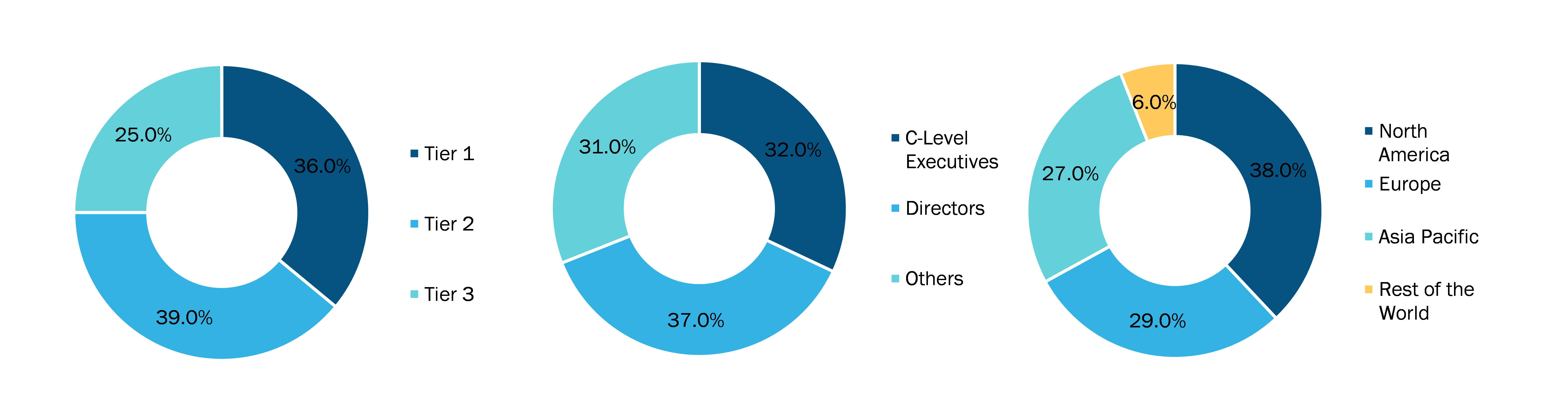
Once we receive the confirmation from primary research sources or primary respondents, we finalize the base year market estimation and forecast the data as per the macroeconomic and microeconomic factors assessed during data collection.
- Data Analysis:
Once data is validated through both secondary as well as primary respondents, we finalize the market estimations by hypothesis formulation and factor analysis at regional and country level.
- Macro-Economic Factor Analysis:
We analyse macroeconomic indicators such the gross domestic product (GDP), increase in the demand for goods and services across industries, technological advancement, regional economic growth, governmental policies, the influence of COVID-19, PEST analysis, and other aspects. This analysis aids in setting benchmarks for various nations/regions and approximating market splits. Additionally, the general trend of the aforementioned components aid in determining the market's development possibilities.
- Country Level Data:
Various factors that are especially aligned to the country are taken into account to determine the market size for a certain area and country, including the presence of vendors, such as headquarters and offices, the country's GDP, demand patterns, and industry growth. To comprehend the market dynamics for the nation, a number of growth variables, inhibitors, application areas, and current market trends are researched. The aforementioned elements aid in determining the country's overall market's growth potential.
- Company Profile:
The “Table of Contents” is formulated by listing and analyzing more than 25 - 30 companies operating in the market ecosystem across geographies. However, we profile only 10 companies as a standard practice in our syndicate reports. These 10 companies comprise leading, emerging, and regional players. Nonetheless, our analysis is not restricted to the 10 listed companies, we also analyze other companies present in the market to develop a holistic view and understand the prevailing trends. The “Company Profiles” section in the report covers key facts, business description, products & services, financial information, SWOT analysis, and key developments. The financial information presented is extracted from the annual reports and official documents of the publicly listed companies. Upon collecting the information for the sections of respective companies, we verify them via various primary sources and then compile the data in respective company profiles. The company level information helps us in deriving the base number as well as in forecasting the market size.
- Developing Base Number:
Aggregation of sales statistics (2020-2022) and macro-economic factor, and other secondary and primary research insights are utilized to arrive at base number and related market shares for 2022. The data gaps are identified in this step and relevant market data is analyzed, collected from paid primary interviews or databases. On finalizing the base year market size, forecasts are developed on the basis of macro-economic, industry and market growth factors and company level analysis.
- Data Triangulation and Final Review:
The market findings and base year market size calculations are validated from supply as well as demand side. Demand side validations are based on macro-economic factor analysis and benchmarks for respective regions and countries. In case of supply side validations, revenues of major companies are estimated (in case not available) based on industry benchmark, approximate number of employees, product portfolio, and primary interviews revenues are gathered. Further revenue from target product/service segment is assessed to avoid overshooting of market statistics. In case of heavy deviations between supply and demand side values, all thes steps are repeated to achieve synchronization.
We follow an iterative model, wherein we share our research findings with Subject Matter Experts (SME’s) and Key Opinion Leaders (KOLs) until consensus view of the market is not formulated – this model negates any drastic deviation in the opinions of experts. Only validated and universally acceptable research findings are quoted in our reports.
We have important check points that we use to validate our research findings – which we call – data triangulation, where we validate the information, we generate from secondary sources with primary interviews and then we re-validate with our internal data bases and Subject matter experts. This comprehensive model enables us to deliver high quality, reliable data in shortest possible time.
 Ottieni un campione gratuito per questo repot
Ottieni un campione gratuito per questo repot