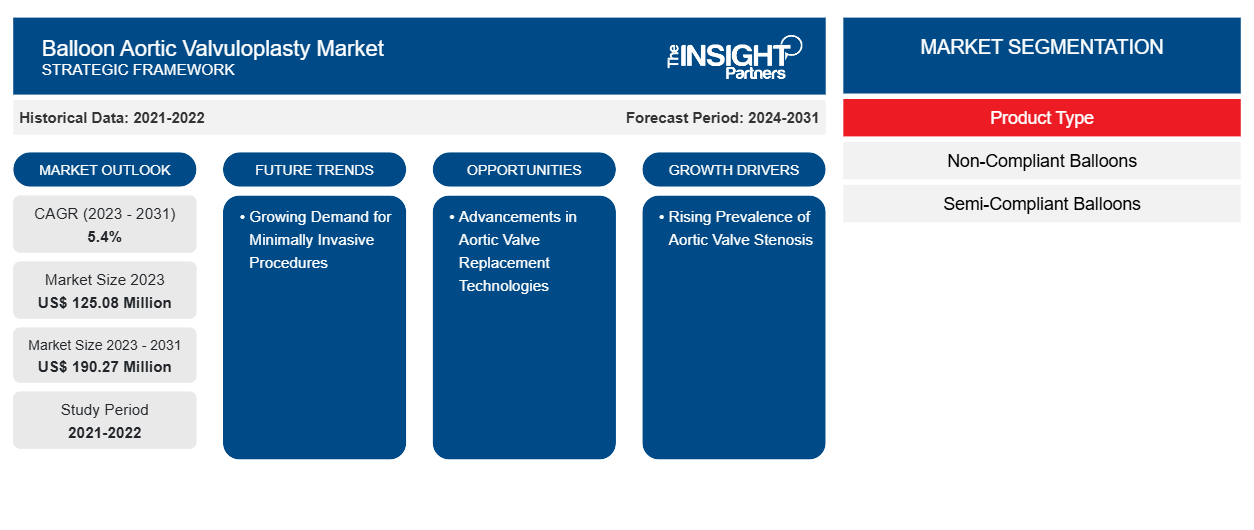
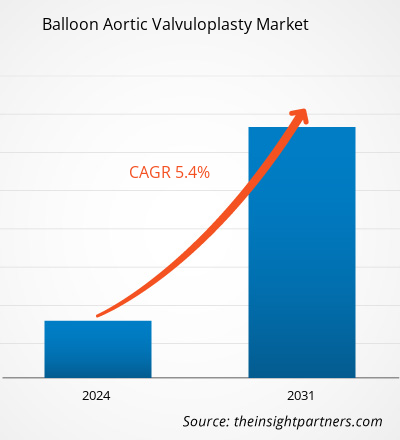
풍선 대동맥 판막 성형술 시장 규모는 2023년 1억 2,508만 달러에서 2031년까지 1억 9,027만 달러로 성장할 것으로 예상됩니다. 이 시장은 2023~2031년 동안 5.4%의 CAGR을 기록할 것으로 예상됩니다. 경피적 대동맥 판막 이식술(TAVI)을 개선하는 데 도움이 되는 새로운 연구 개발 전략이 시장의 주요 트렌드로 남을 가능성이 높습니다.
풍선 대동맥 판막 성형술 시장 분석
풍선 판막성형술 장치 시장 성장을 견인하는 요인에는 급속한 고령화 인구와 대동맥 판막 협착증의 유병률 증가가 포함됩니다. 대동맥 협착증은 수술적 치료가 필요한 가장 흔한 판막 질환이 되었습니다. 풍선 대동맥 판막성형술(BAV)은 대동맥 협착증이 있는 광범위한 환자에게 도움이 될 수 있으며, TAVI 시술에서의 역할 외에도 판막 교체에 대한 브리징 요법이 될 수 있습니다. 풍선 판막성형술 장치에 대한 의료 보상의 가용성 증가, 최소 침습 수술의 채택 증가, 더 나은 의료 인프라는 시장 성장에 기여하는 추가 요인입니다. 최소 침습적 접근 방식은 일반적으로 심장에 접근하기 위한 특수 도구와 기술을 사용하여 더 작은 절개를 수반하므로 입원 기간이 단축되고 흉터가 덜 남을 수 있습니다. 또한, 의료 기관은 의료팀을 교육하고 최소 침습적 BAV 및 TAVI 수술에 대한 증가하는 수요를 충족하기 위해 필요한 장비를 확보하는 데 투자하여 환자에게 향상된 치료 옵션과 더 나은 전반적인 의료 경험을 제공하고 있습니다. 따라서 풍선 대동맥 판막 성형술 시장은 주목할 만한 성장을 목격합니다.
풍선 대동맥 판막 성형술 시장 개요
심각한 심장판막 질환을 앓고 있는 환자의 절반 이상이 증상이 나타난 지 2년 이내에 사망합니다. Global Heart Hub에서 Heart Valve Voice Canada 2020에 발표한 설문 조사 결과에 따르면 60세 이상의 캐나다인 중 대동맥 협착증을 알고 있는 사람은 3%에 불과합니다. 따라서 심장판막 질환에 대한 인식을 높이고 조기에 발견하는 것은 환자와 환자에 의존하는 사람, 지역 사회에 필수적입니다. Canadian Cardiovascular Society는 캐나다에서 심장판막 질환 치료의 격차를 해소하기 위해 심장판막 질환 전략 실무 그룹을 구성했습니다. 2021년 1월, Medtronic은 Health Canada로부터 Evolut TAVI 시스템에 대한 확대된 적응증을 받았습니다. Medtronic의 TAVI 플랫폼은 이첨판 대동맥 판막(중간 또는 고위험 환자)에 대해 허가된 유일한 시스템입니다. Evolut은 모든 위험 범주에 걸쳐 심각한 대동맥 협착증 환자에게 국가에서 적응증을 받았습니다. 2021년 9월, Abbott은 증상이 있고 심각한 대동맥판 협착증이 있으며 개흉 수술 위험이 높은 환자를 치료하기 위해 Portico with FlexNav 경피적 대동맥판판판막 교체(TAVR) 시스템에 대한 미국 식품의약국(FDA) 승인을 받았습니다.
귀하의 요구 사항에 맞게 이 보고서를 사용자 정의하세요
이 보고서의 일부 또는 국가 수준 분석, Excel 데이터 팩을 포함하여 모든 보고서에 대한 사용자 정의를 무료로 받을 수 있으며 신생 기업 및 대학을 위한 훌륭한 혜택과 할인 혜택을 이용할 수 있습니다.
- 이 보고서의 주요 시장 동향을 알아보세요.이 무료 샘플에는 시장 동향부터 추정 및 예측까지 다양한 데이터 분석이 포함됩니다.
풍선 대동맥 판막 성형술 시장 동인 및 기회
최소 침습적 시술에 대한 수요 증가로 시장 성장이 촉진됩니다.
판막 교체 수술에서 최소 침습적 기술에 대한 수요가 급증하는 것은 의료 관행과 환자 선호도의 상당한 변화를 반영합니다. 최소 침습적 대동맥 판막 성형술에서 심장의 대동맥 판막은 수리되고 필요한 경우 TAVI 절차를 통해 교체됩니다. 최소 침습적 기술은 절차의 침습성을 줄이고 관련 위험을 최소화하며 더 빠른 회복 시간을 촉진하려는 욕구에 의해 주도됩니다.
심장학을 포함한 모든 의학 분야에서는 전통적인 개심술에 비해 여러 가지 이점이 있기 때문에 덜 침습적인 시술에 대한 수요가 증가하고 있습니다. 개심술에 비해 최소 침습적 시술을 받은 환자는 일반적으로 회복 시간이 짧습니다. 또한 최소 침습적 기술은 합병증 위험이 낮고 개심술에 비해 흉터가 적습니다. 외과의는 의료 기기의 발전으로 이러한 기술을 채택했으며, 향상된 수술 기술은 광범위한 채택을 더욱 촉진할 수 있습니다. BAV는 대동맥 판막 협착증을 포함한 다양한 심장 문제를 치료하는 데 사용할 수 있는 최소 침습적 기술이기 때문에 BAV 장치 시장은 최소 침습적 시술에 대한 수요 증가로 인해 주도되고 있습니다.
대동맥 판막 교체 기술의 발전
심장 질환이 있는 환자는 수술적 개입에 비해 경피적 시술로 치료할 경우 더 짧은 기간 내에 증상이 개선됩니다. TAVI와 같은 최소 침습 수술 기술은 발전으로 인해 대동맥 협착증 치료를 현대화했습니다. 또한, 설계 개선으로 더 강하고 생체적합성이 있는 대동맥 판막 교체술이 개발되어 환자 결과가 향상되고 합병증 위험이 낮아졌습니다. 풍선 대동맥 판막 성형술 및 대동맥 판막 교체 장치 분야에서 회사가 이룬 몇 가지 기술적 발전은 다음과 같습니다.
- 2023년 1월, Abbott은 심각한 대동맥 협착증 환자와 개심술이 불가능한 환자를 치료하기 위한 Navitor TAVI 시스템에 대한 FDA 승인을 받았습니다. Navitor는 회사의 광범위한 경피적 구조적 심장 포트폴리오에 새롭게 도입되었으며, 의사와 환자에게 다양한 심각한 심장 질환에 대한 덜 침습적인 치료 대안을 제공합니다.
- 2020년 9월, Boston Scientific은 유럽에서 ACURATE neo2 대동맥 판막 시스템을 출시했습니다. 차세대 TAVI 기술(원래 ACURATE neo 플랫폼의 임상 성능을 개선하기 위해 여러 기능을 갖춘 새로운 플랫폼)은 이전 세대의 대동맥 판막 시스템에 비해 대동맥 협착증 환자에게 더 광범위한 적응증을 제공했습니다.
따라서 기술의 발전은 풍선 대동맥 판막 성형술 시장 성장에 수익성 있는 기회를 제공하고 있습니다.
풍선 대동맥 판막 성형술 시장 보고서 세분화 분석
풍선 대동맥 판막 성형술 시장 분석에 기여한 핵심 세그먼트는 제품 유형입니다.
제품 유형에 따라 풍선 대동맥 판막 성형술 시장은 비준수 풍선과 반준수 풍선으로 나뉩니다. 비준수 풍선 세그먼트는 2023년에 더 큰 시장 점유율을 차지했으며 2023~2031년 동안 시장에서 더 높은 CAGR을 기록할 것으로 예상됩니다.
지역별 풍선 대동맥 판막 성형술 시장 점유율 분석
풍선 대동맥 판막 성형술 시장 보고서의 지리적 범위는 주로 북미, 아시아 태평양, 유럽, 중동 및 아프리카, 남미 및 중부 아메리카의 5개 지역으로 나뉩니다.
북미는 시장에서 상당한 점유율을 차지했습니다. 이 지역의 시장 성장은 심장 판막 질환의 유병률 증가, 인구 사이에서 심장 판막 질환에 대한 인식을 높이기 위한 다양한 기관의 이니셔티브 증가, 치료 장비의 발전에 기인합니다. 경제 협력 개발 기구에 따르면 멕시코에서 심혈관 질환의 부담이 빠르게 증가하고 있습니다. 성인 및 노인 인구의 심장 질환과 관련된 위험 요소는 인식 프로그램을 통해 멕시코에서 해결되고 있습니다. 이러한 인식 프로그램에 대응하여 경피적 대동맥 판막 교체 수술이 인기를 얻었으며 일반적으로 개심판 수술의 대안으로 선호됩니다. 멕시코시티의 심장 전문의는 최고의 기술과 기법을 사용하여 TAVI를 수행합니다. CMQ 병원은 푸에르토 발라르타와 리비에라 나야리트에서 TAVI 시술을 선도하고 있습니다. 이 병원은 중재적 심장학 분야의 전문가로 구성된 다학제 팀을 보유하고 있어 각 환자에게 포괄적이고 개인화된 치료를 보장합니다.
캐나다에서 대동맥판 협착증의 가장 흔한 원인은 노화로 인한 퇴행성 판막 질환이며, 그 다음으로 선천성 이첨판 대동맥판 질환과 류마티스성 심장 질환이 뒤따릅니다. 2022년 3월 Institute of Health Economics에서 발표한 "캐나다의 심장판막 질환"에 따르면 대동맥판 협착증과 승모판 역류증이 가장 흔한 두 가지 심장판막 질환입니다. 국가 인구의 약 2.5%가 심장판막 질환을 앓고 있는 것으로 보고되었으며, 65세 이후에는 상당히 증가하여 75세 이상의 경우 13%에 이릅니다. 캐나다에서는 2040년까지 65세 이상의 150만 명이 심장판막 질환을 앓을 것으로 추산됩니다.
캐나다에서는 상당수의 시장 참여자가 TAVI 제품을 제공합니다. Edwards Lifesciences, Boston Scientific Corporation, Medtronic Inc., JenaValve Technology, Transcatheter Technologies GmbH는 시장에서 운영되는 몇몇 제조업체입니다. 이러한 제조업체는 캐나다 정부로부터 규제 승인을 받았습니다. Edwards SAPIEN 및 Medtronic CoreValve 시스템은 캐나다에서 라이선스를 받았습니다.
풍선 대동맥 판막 성형술 시장 지역 통찰력
Insight Partners의 분석가들은 예측 기간 동안 풍선 대동맥 판막 성형술 시장에 영향을 미치는 지역적 추세와 요인을 철저히 설명했습니다. 이 섹션에서는 또한 북미, 유럽, 아시아 태평양, 중동 및 아프리카, 남미 및 중미의 풍선 대동맥 판막 성형술 시장 세그먼트와 지리에 대해서도 설명합니다.
- 풍선 대동맥 판막 성형술 시장에 대한 지역별 데이터 얻기
풍선 대동맥 판막 성형술 시장 보고서 범위
| 보고서 속성 | 세부 |
|---|---|
| 2023년 시장 규모 | 1억 2,508만 달러 |
| 2031년까지 시장 규모 | 1억 9,027만 달러 |
| 글로벌 CAGR (2023-2031) | 5.4% |
| 역사적 데이터 | 2021-2022 |
| 예측 기간 | 2024-2031 |
| 다루는 세그먼트 | 제품 유형별
|
| 포함된 지역 및 국가 | 북아메리카
|
| 시장 선도 기업 및 주요 회사 프로필 |
|
풍선 대동맥 판막 성형술 시장 참여자 밀도: 비즈니스 역학에 미치는 영향 이해
풍선 대동맥 판막 성형술 시장은 소비자 선호도의 변화, 기술 발전, 제품의 이점에 대한 인식 증가와 같은 요인으로 인해 최종 사용자 수요가 증가함에 따라 빠르게 성장하고 있습니다. 수요가 증가함에 따라 기업은 제품을 확장하고, 소비자의 요구를 충족하기 위해 혁신하고, 새로운 트렌드를 활용하여 시장 성장을 더욱 촉진하고 있습니다.
시장 참여자 밀도는 특정 시장이나 산업 내에서 운영되는 회사나 기업의 분포를 말합니다. 주어진 시장 공간에 얼마나 많은 경쟁자(시장 참여자)가 존재하는지 그 규모나 총 시장 가치에 비해 나타냅니다.
풍선 대동맥 판막 성형술 시장에서 운영되는 주요 회사는 다음과 같습니다.
- B 브라운 SE
- TT 메디컬 주식회사
- 발튼
- 벡튼 딕킨슨 앤 코
- 에드워즈 라이프사이언스 코퍼레이션
- 발트
면책 조항 : 위에 나열된 회사는 어떤 특별한 순서에 따라 순위가 매겨지지 않았습니다.
- 풍선 대동맥 판막 성형술 시장 주요 주요 업체 개요를 알아보세요
풍선 대동맥 판막 성형술 시장 소식 및 최근 동향
풍선 대동맥 판막 성형술 시장은 1차 및 2차 연구 이후의 정성적, 정량적 데이터를 수집하여 평가합니다. 여기에는 중요한 기업 간행물, 협회 데이터 및 데이터베이스가 포함됩니다. 풍선 대동맥 판막 성형술 시장의 몇 가지 개발 사항은 다음과 같습니다.
- Abbott은 인도에서 최신 세대 TAVI 시스템인 Navitor를 출시하여, 이 최소 침습적 장치를 인도에서 수술 위험이 높거나 극심한 심각한 대동맥 협착증 환자에게 사용할 수 있게 했습니다. Navitor는 심장 주기와 함께 작동하여 판막 주위 누출이라고 알려진 판막 프레임을 가로지르는 혈액 역류를 최소화하거나 제거하기 위해 고유한 패브릭 커프(NaviSeal)를 특징으로 합니다. (출처: Abbott, 보도자료, 2022년 12월)
- Keystone Heart, Ltd.는 InterValve Medical Inc.와 독점 유통 계약을 체결하여 미국에서 풍선 대동맥 판막 성형술 제품 포트폴리오를 판매하고 마케팅했습니다. 이 계약에 따라 Keystone Heart Ltd.는 미국 시장에서 V8 및 TAV8 대동맥 판막 성형술 풍선 카테터(InterValve Medical) 판매를 시작했습니다. (출처: Keystone Heart, Ltd; 보도자료, 2021년 3월)
풍선 대동맥 판막 성형술 시장 보고서 범위 및 제공물
“풍선 대동맥 판막 성형술 시장 규모 및 예측(2021-2031)” 보고서는 아래 영역을 포괄하는 시장에 대한 자세한 분석을 제공합니다.
- 범위에 포함된 모든 주요 시장 세그먼트에 대한 글로벌, 지역 및 국가 수준의 풍선 대동맥 판막 성형술 시장 규모 및 예측
- 풍선 대동맥 판막 성형술 시장 동향 및 드라이버, 제약 및 주요 기회와 같은 시장 역학
- 자세한 PEST 및 SWOT 분석
- 주요 시장 동향, 글로벌 및 지역 프레임워크, 주요 업체, 규정 및 최근 시장 개발 사항을 포괄하는 풍선 대동맥 판막 성형술 시장 분석
- 시장 집중도, 히트맵 분석, 유명 업체 및 풍선 대동맥 판막 성형술 시장의 최근 개발 사항을 다루는 산업 환경 및 경쟁 분석
- 자세한 회사 프로필
- 역사적 분석(2년), 기준 연도, CAGR을 포함한 예측(7년)
- PEST 및 SWOT 분석
- 시장 규모 가치/양 - 글로벌, 지역, 국가
- 산업 및 경쟁 환경
- Excel 데이터 세트

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends
Segment Covered
This text is related
to segments covered.

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America
Country Scope
This text is related
to country scope.
자주 묻는 질문
The estimated value of the balloon aortic valvuloplasty market can reach US$ 190.27 million by 2031.
North America region dominated the balloon aortic valvuloplasty market in 2023.
B Braun SE, TT Medical, Inc., Balton, Becton Dickinson and Co, Edwards Lifesciences Corp, Balt, Venus MedTech HangZhou Inc., NuMED, simeks, and OSYPKA are among the key market players in the balloon aortic valvuloplasty market.
The global balloon aortic valvuloplasty market is estimated to register a CAGR of 5.4% during the forecast period 2023–2031.
The rising prevalence of aortic valve stenosis and increasing demand for minimally invasive procedures are the most influential factors responsible for market growth.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Balloon Aortic Valvuloplasty Market
- B Braun SE
- TT Medical, Inc.
- Balton
- Becton Dickinson and Co
- Edwards Lifesciences Corp
- Balt
- Venus MedTech HangZhou Inc
- NuMED
- simeks
- OSYPKA
The Insight Partners performs research in 4 major stages: Data Collection & Secondary Research, Primary Research, Data Analysis and Data Triangulation & Final Review.
- Data Collection and Secondary Research:
As a market research and consulting firm operating from a decade, we have published and advised several client across the globe. First step for any study will start with an assessment of currently available data and insights from existing reports. Further, historical and current market information is collected from Investor Presentations, Annual Reports, SEC Filings, etc., and other information related to company’s performance and market positioning are gathered from Paid Databases (Factiva, Hoovers, and Reuters) and various other publications available in public domain.
Several associations trade associates, technical forums, institutes, societies and organization are accessed to gain technical as well as market related insights through their publications such as research papers, blogs and press releases related to the studies are referred to get cues about the market. Further, white papers, journals, magazines, and other news articles published in last 3 years are scrutinized and analyzed to understand the current market trends.
- Primary Research:
The primarily interview analysis comprise of data obtained from industry participants interview and answers to survey questions gathered by in-house primary team.
For primary research, interviews are conducted with industry experts/CEOs/Marketing Managers/VPs/Subject Matter Experts from both demand and supply side to get a 360-degree view of the market. The primary team conducts several interviews based on the complexity of the markets to understand the various market trends and dynamics which makes research more credible and precise.
A typical research interview fulfils the following functions:
- Provides first-hand information on the market size, market trends, growth trends, competitive landscape, and outlook
- Validates and strengthens in-house secondary research findings
- Develops the analysis team’s expertise and market understanding
Primary research involves email interactions and telephone interviews for each market, category, segment, and sub-segment across geographies. The participants who typically take part in such a process include, but are not limited to:
- Industry participants: VPs, business development managers, market intelligence managers and national sales managers
- Outside experts: Valuation experts, research analysts and key opinion leaders specializing in the electronics and semiconductor industry.
Below is the breakup of our primary respondents by company, designation, and region:
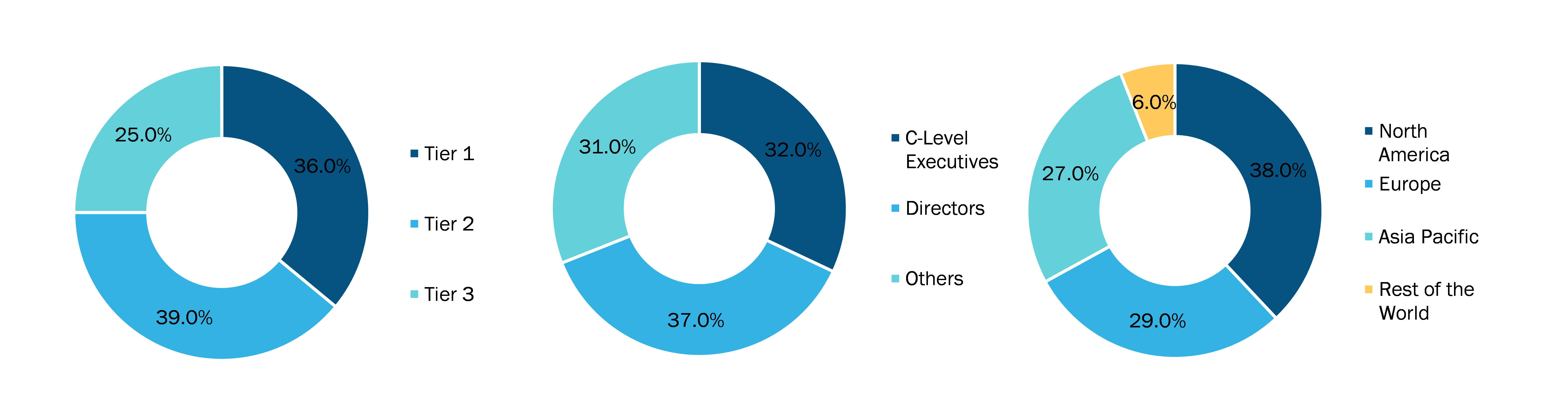
Once we receive the confirmation from primary research sources or primary respondents, we finalize the base year market estimation and forecast the data as per the macroeconomic and microeconomic factors assessed during data collection.
- Data Analysis:
Once data is validated through both secondary as well as primary respondents, we finalize the market estimations by hypothesis formulation and factor analysis at regional and country level.
- Macro-Economic Factor Analysis:
We analyse macroeconomic indicators such the gross domestic product (GDP), increase in the demand for goods and services across industries, technological advancement, regional economic growth, governmental policies, the influence of COVID-19, PEST analysis, and other aspects. This analysis aids in setting benchmarks for various nations/regions and approximating market splits. Additionally, the general trend of the aforementioned components aid in determining the market's development possibilities.
- Country Level Data:
Various factors that are especially aligned to the country are taken into account to determine the market size for a certain area and country, including the presence of vendors, such as headquarters and offices, the country's GDP, demand patterns, and industry growth. To comprehend the market dynamics for the nation, a number of growth variables, inhibitors, application areas, and current market trends are researched. The aforementioned elements aid in determining the country's overall market's growth potential.
- Company Profile:
The “Table of Contents” is formulated by listing and analyzing more than 25 - 30 companies operating in the market ecosystem across geographies. However, we profile only 10 companies as a standard practice in our syndicate reports. These 10 companies comprise leading, emerging, and regional players. Nonetheless, our analysis is not restricted to the 10 listed companies, we also analyze other companies present in the market to develop a holistic view and understand the prevailing trends. The “Company Profiles” section in the report covers key facts, business description, products & services, financial information, SWOT analysis, and key developments. The financial information presented is extracted from the annual reports and official documents of the publicly listed companies. Upon collecting the information for the sections of respective companies, we verify them via various primary sources and then compile the data in respective company profiles. The company level information helps us in deriving the base number as well as in forecasting the market size.
- Developing Base Number:
Aggregation of sales statistics (2020-2022) and macro-economic factor, and other secondary and primary research insights are utilized to arrive at base number and related market shares for 2022. The data gaps are identified in this step and relevant market data is analyzed, collected from paid primary interviews or databases. On finalizing the base year market size, forecasts are developed on the basis of macro-economic, industry and market growth factors and company level analysis.
- Data Triangulation and Final Review:
The market findings and base year market size calculations are validated from supply as well as demand side. Demand side validations are based on macro-economic factor analysis and benchmarks for respective regions and countries. In case of supply side validations, revenues of major companies are estimated (in case not available) based on industry benchmark, approximate number of employees, product portfolio, and primary interviews revenues are gathered. Further revenue from target product/service segment is assessed to avoid overshooting of market statistics. In case of heavy deviations between supply and demand side values, all thes steps are repeated to achieve synchronization.
We follow an iterative model, wherein we share our research findings with Subject Matter Experts (SME’s) and Key Opinion Leaders (KOLs) until consensus view of the market is not formulated – this model negates any drastic deviation in the opinions of experts. Only validated and universally acceptable research findings are quoted in our reports.
We have important check points that we use to validate our research findings – which we call – data triangulation, where we validate the information, we generate from secondary sources with primary interviews and then we re-validate with our internal data bases and Subject matter experts. This comprehensive model enables us to deliver high quality, reliable data in shortest possible time.
 이 보고서에 대한 무료 샘플을 받으세요
이 보고서에 대한 무료 샘플을 받으세요