Cervical Intraepithelial Neoplasia 3 Segment to Lead Global CIN and HR-HPV Treatment Market During 2023–2028
According to our new research study on “CIN and HR-HPV Treatment Market Forecast to 2028 – COVID-19 Impact and Global Analysis – by Disease Type, Strain Type, Offering, Product Type, and End User,” the CIN and HR-HPV Treatment Market was valued at US$ 11,635.17 million in 2022 and is projected to reach US$ 17,164.61 million by 2028; it is estimated to grow at a CAGR of 6.7% from 2023 to 2028. Factors driving the growth of the CIN and HR-HPV Treatment Market include an increase in the prevalence of human papillomavirus (HPV) infections and favorable initiatives for preventing cervical cancer.
Based on offering, the CIN and HR-HPV Treatment Market is segmented into cervical intraepithelial neoplasia 1, cervical intraepithelial neoplasia 2, and cervical intraepithelial neoplasia 3. The cervical intraepithelial neoplasia 3 segment held the largest share of the market in 2022 and is anticipated to register a CAGR of 7.6% in the market during the forecast period. According to the American Society for Colposcopy and Cervical Pathology (ASCCP), in the US, the estimated spontaneous regression rate for CIN 3 in 2023 is 32–47%, and if the condition remains untreated, 12–40% of cases are likely to progress into invasive cancer. Conization is widely accepted as the therapy of choice for treating CIN 3. Further, ~0.2–4% of cervical cancer cases that are detected as CIN 3 transform into invasive cervical cancer within the first year. HPV-16 and HPV-18 cause ~50% of CIN 3 and 70% of cervical cancer cases every year. It is recommended that women with CIN 3 should undergo treatment, as there is a low rate of spontaneous regression and a higher rate of progression. Both ablative and excisional procedures can potentially cure most patients with CIN 3. Such aforementioned factors contribute to the overall CIN and HR-HPV Treatment Market size and growth during the forecast period.
CIN & hrHPV Market, by Region, 2022 (%)
CIN & HR-HPV Treatment Market Forecast to 2028 - Global Analysis By Disease Type (Cervical Intraepithelial Neoplasia 1, Cervical Intraepithelial Neoplasia 2, and Cervical Intraepithelial Neoplasia 3), Strain Type (HPV 16, HPV 18, and Others), Offering (Diagnostic Method and Treatment), Product Type (Kits & Reagents, Instruments, and Services), and End User (Hospitals & Clinics, Diagnostic Laboratories, Specialized Clinical Laboratories, and Others)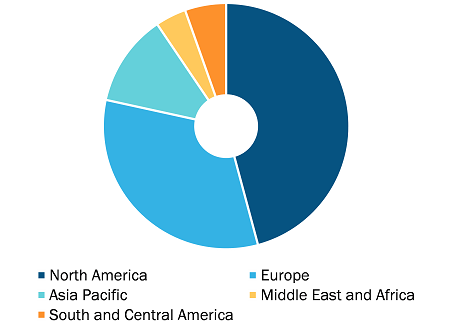
CIN & HR-HPV Treatment Market SWOT Analysis by 2028
Download Free Sample
Impact of COVID-19 Pandemic on CIN & hrHPV Market
Due to the fear of COVID-19 infection, women hesitated to undergo cervical cancer testing, which is performed using PAP tests, biopsies, and colonoscopy, as it generally requires paying visits to healthcare facilities. This trend among patients affected the cervical cancer diagnostic testing market in the region. However, healthcare providers are now offering at-home testing services for the initial diagnosis through point-of-care devices and kits. Companies in the CIN and HR-HPV Treatment Market are actively involved in organic and inorganic developments. In April 2020 F. Hoffmann-La Roche Ltd received US Food and Drug Administration (FDA) approval for the cobas HPV test for use on the fully automated, high-throughput cobas 6800/8800 Systems. It helps identify women at risk of cervical cancer by detecting the presence of high-risk human papillomavirus (HPV) DNA in cervical samples.
Fujirebio Europe NV, Qiagen NV, Zilico Ltd, Abbott Laboratories, Cepheid, F. Hoffmann-La Roche Ltd, INOVIO Pharmaceuticals Inc, Bioneer Corp, Antiva Biosciences Inc, and Thermo Fisher Scientific Inc are among the leading companies operating in the CIN & hrHPV market.
Based on offering, the CIN and HR-HPV Treatment Market is segmented into cervical intraepithelial neoplasia 1, cervical intraepithelial neoplasia 2, and cervical intraepithelial neoplasia 3. The cervical intraepithelial neoplasia 3 segment held the largest market share in 2022, and it is anticipated to register the highest CAGR during the forecast period. Based on strain type, the CIN and HR-HPV Treatment Market is segmented into HPV 16, HPV 18, and others. The HPV 18 segment held the largest share of the market in 2022, and the same segment is expected to register the highest CAGR during the forecast period. By offering, the CIN and HR-HPV Treatment Market is bifurcated into diagnostic method and treatment. By product type, the market is segmented into kits & reagents, instruments, and services. By end user, the CIN and HR-HPV Treatment Market is segmented as hospitals & clinics, diagnostic laboratories, specialized clinical laboratories, and others. By geography, the CIN and HR-HPV Treatment Market is segmented into North America (US, Canada, and Mexico), Europe (France, Germany, UK, Spain, Italy, and Rest of Europe), Asia Pacific (China, India, Japan, Australia, South Korea, and Rest of Asia Pacific), Middle East & Africa (Saudi Arabia, UAE, South Africa, and Rest of Middle East & Africa), and South & Central America (Brazil, Argentina, and Rest of South & Central America).
In terms of revenue, North America dominated the CIN and HR-HPV Treatment Market growth accounting highest market share. On the other hand, Asia Pacific accounts for highest CAGR for CIN and HR-HPV Treatment Market during the forecast period.
Contact Us
Phone: +1-646-491-9876
Email Id: sales@theinsightpartners.com