Service segment by component is expected to grow at a fast pace over the forecast period
According to The Insight Partners market research study of 'Electronic Trial Master File (eTMF) Market to 2027 - Global Analysis and Forecasts by Component, Delivery Mode, and End-User'. The global electronic trial master file market is expected to reach US$ 3,155.64 Mn in 2027 from US$ 938.32 Mn in 2019. The market is estimated to grow with a CAGR of 16.5% from 2020-2027. The report provides trends prevailing in the global electronic trial master file (eTMF) market and the factors driving market along with those that act as hindrances.
The global electronic trial master file (eTMF) market, based on the component, is segmented into software and service. The service segment held the largest share of the market in 2019. Moreover, the same segment is anticipated to register the highest CAGR of 17.0% in the market during the forecast period. The clinical trial process is a very complex and highly regulated stage for pharmaceutical, biotechnology, and other life science companies. eTMF solutions enable these companies to manage their workflow efficiently and accurately. The development of information technology has allowed healthcare IT companies to offers solutions and services for clinical trial administration.
Global Electronic Trial Master File (eTMF) Market, By Regions, 2019 (%)
Electronic Trial Master File (eTMF) Market to 2027 - Global Analysis and Forecasts By Component (Service, Software); Delivery Mode (Cloud-Based eTMF, On-Premise eTMF), End-User (Pharmaceutical and Biotechnology Companies, CROs, Others); and Geography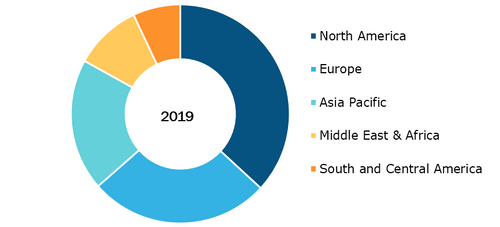
Electronic Trial Master File (eTMF) Market Strategies - 2027
Download Free Sample
The market for electronic trial master file (eTMF) is expected to grow, owing to factors such as rising in number of clinical trials and rising adoption of eTMF. Moreover, strategic initiatives by market players are likely to have a positive impact on the growth of the market in coming years. Besides, digitalization of clinical trials is expected to be a prevalent trend in the market.
Leading companies operating in the electronic trial master file (eTMF) market are Covance Inc (Lab Corp), Oracle, Ennov, Mastercontrol, Inc., Omnicomm, Pharmavigilalnce, Veeva Systems, and Phlexglobal, Aurea, Inc and TRANSPERFECT among others.
The report segments Global Electronic Trial Master File Market as follows:
Global Electronic Trial Master File (eTMF) Market - By Component
- Service
- Software
Global Electronic Trial Master File (eTMF) Market - By Delivery Mode
- Cloud-Based
- On-Premises
Global Electronic Trial Master File (eTMF) Market - By End User
- Pharmaceutical and Biotechnology Companies
- CROs
- Others
Global Electronic Trial Master File (eTMF) Market - By Geography
- North America
- US
- Canada
- Mexico
- Europe
- France
- Germany
- U.K
- Spain
- Italy
- Asia Pacific (APAC)
- China
- India
- Japan
- Australia
- South Korea
- Middle East & Africa (MEA)
- Saudi Arabia
- U.A.E
- South Africa
- South & Central America (SCAM)
- Brazil
- Argentina
Contact Us
Phone: +1-646-491-9876
Email Id: sales@theinsightpartners.com