According to our new research study on “Lyophilization Services for Biopharmaceuticals Market –Global Analysis and Forecast – by Service Type and End User,” the market is expected to reach US$ 3,586.55 million by 2028 from US$ 2,051.41 million in 2022; it is estimated to record a CAGR of 9.9% from 2023 to 2028. The report highlights the trends prevalent in the global lyophilization services for biopharmaceuticals market, and the drivers and deterrents pertaining to its growth.
Based on service type, the lyophilization services for biopharmaceuticals market is segmented into lyophilization cycle development, clinical manufacturing, commercial manufacturing, and freeze drying analytical services. The commercial manufacturing segment held the largest market share in 2022. However, the lyophilization cycle development segment is anticipated to record a significant growth rate during the forecast period. The lyophilization services for biopharmaceuticals market growth is attributed to the advantages offered by the lyophilization process, including increased shelf life of small and large molecule drugs, stabilizing formulation by commercially validated methods, and reducing the cost of complex logistics such as rigorous cold-chain custody validation regimes and constant documentable refrigeration at the dispensary level.
Global Lyophilization Services for Biopharmaceuticals Market, by Region, 2022 (%)
Lyophilization Services for Biopharmaceuticals Market Forecast to 2028 - Global Analysis By Service Type (Lyophilization Cycle Development, Clinical Manufacturing, Commercial Manufacturing, and Freeze Drying Analytical Services), and End User (Pharmaceutical & Biotechnology Companies, Research Institutes, and Others)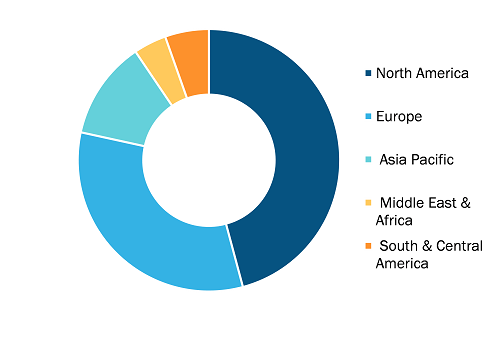
Lyophilization Services for Biopharmaceuticals Market 2028
Download Free Sample
Impact of COVID-19 Pandemic on Lyophilization Services for Biopharmaceuticals Market.
Lyophilization is an effective technique that researchers are using as the scientific community aims to stabilize the SARS-CoV-2 virus vaccines, such as mRNA-LNP SARS-CoV-2 vaccines. According to an article published in the National Library of Medicine in April 2021, can , lyophilization is expected to be a logical step to improve the long-term stability of mRNA-LNP formulations. The head of Pfizer's viral vaccine research, Philip Dormitzer, has already mentioned Pfizer’s aspiration to use lyophilization for mRNA-LNP SARS-CoV-2 vaccines. Moreover, a lyophilized form of an mRNA-based cytomegalovirus vaccine (mRNA-1647) is used in phase 2 clinical trials. It has a claimed shelf life of ≥ 18 months at 5 °C. Service providers in the lyophilization service for Biopharmaceuticals market experienced increased demand, mostly for researching and developing COVID-19 vaccines or therapeutics. Hence, the outbreak of COVID-19 has positively impacted the lyophilization service for Biopharmaceuticals market.
ATTWILL Medical Solutions, Axcellerate Pharma LLC, Labyrinth Biopharma LLC, Berkshire Sterile Manufacturing, PCI Pharma Services, Curia Global Inc, Emergent BioSolutions Inc., Jubilant HollisterStier LLC, Biofortuna, Lyophilization Technology Inc., and SYNERLAB GROUP are among the prominent players operating in the lyophilization services for biopharmaceuticals market. The market players are adopting organic strategies to sustain their position in the lyophilization services for biopharmaceuticals market. In February 2022, Lyophilization Technology, Inc. (LTI) successfully developed innovative technology for processing lyophilized products in dual-chamber cartridges and syringes. The unique and novel aspects of the innovation provide for processing a wide variety of products. These products span small molecules, biologics such as proteins, and vaccines.
Lyophilization Services for Biopharmaceuticals Market: Competitive Landscape and Key Developments
ATTWILL Medical Solutions, Axcellerate Pharma LLC, Labyrinth Biopharma LLC, Berkshire Sterile Manufacturing, PCI Pharma Services, Curia Global Inc, Emergent BioSolutions Inc., Jubilant HollisterStier LLC, Biofortuna, Lyophilization Technology Inc., and SYNERLAB GROUP are a few key companies operating in the lyophilization services for biopharmaceuticals market. These companies adopt product innovation strategies to meet evolving customer demands, which allows them to maintain their brand name.
A few of the recent developments in the global lyophilization services for biopharmaceuticals market are mentioned below:
- In May 2022, PCI Pharma Services (PCI), a leading global CDMO, announced a major expansion of capacity and capabilities in sterile lyophilization technology and aseptic liquid fill-finish, an important manufacturing process commonly used with injectable and biologic therapies, with the investment of US$ 100 million in the construction and enhancement of world-class facilities at its Bedford, New Hampshire campus. The facility will contain state-of-the-art technology, including an aseptic fill-finish line with a completely isolated containment system..
- In November 2022, Lyophilization Technology, Inc. (LTI) successfully developed innovative technology to process lyophilized products in dual-chamber cartri
- dges and syringes. The unique and novel aspects of the innovation provide processing for a wide variety of products. These products span small molecules, biologics such as proteins and vaccines.
- In February 2022, Berkshire Sterile Manufacturing (BSM) announced the addition of formulation, lyophilization, and method development capabilities to complement their clients’ drug productions. BSM will offer first-in-human formulation development, supply material for preclinical studies, and conduct research-level stability studies. It will also offer formulation optimization, process development, process optimization, and scale-up studies.
The report segments the global lyophilization services for biopharmaceuticals market as follows:
Based on service type, the lyophilization services for biopharmaceuticals market is segmented into lyophilization cycle development, clinical manufacturing, commercial manufacturing, and freeze drying analytical services. By end user, the lyophilization services for biopharmaceuticals market is segmented into pharmaceutical & biotechnology companies, research institutes, and others. By geography, the Lyophilization Services for Biopharmaceuticals market is segmented into North America (the US, Canada, and Mexico), Europe (France, Germany, the UK, Spain, Italy, and the Rest of Europe), Asia Pacific (China, India, Japan, Australia, South Korea, and the Rest of APAC), the Middle East & Africa (Saudi Arabia, the UAE, South Africa, and the Rest of MEA), and South & Central America (Brazil, Argentina, and the Rest of South & Central America).
Contact Us
Phone: +1-646-491-9876
Email Id: sales@theinsightpartners.com