Laboratory Testing to Dominate Molecular Diagnostic for Infectious Diseases Market Based on Application During 2022–2030
According to our new research study on "Molecular Diagnostic for Infectious Diseases Market Forecast to 2030 – Global Analysis – by Type, Disease Type, Infection Type, Application, and End User," the market was valued at US$ 6,883.03 million in 2022 and is projected to reach US$ 17,614.44 million by 2030. It is estimated to register a CAGR of 12.5% during 2022–2030.
Based on application, the molecular diagnostic for infectious diseases market is bifurcated into point-of-care testing and laboratory testing. Point-of-care testing is further segmented into detection of a single pathogen, detection of two or more pathogens, evaluation of emerging novel infections, surveillance and early detection of biothreat agents and diseases-related biomarkers, and antimicrobial resistance profiling. Laboratory testing is further segmented into patient stratification, drug regimen selection, toxicity avoidance, therapeutic monitoring, and detection of predisposition to disease. Laboratory testing applications include patient stratification, drug regimen selection, toxicity avoidance, therapeutic monitoring, and detection of predisposition to disease. Recognizing diseases through accurate proteomic and genomic biomarkers detection in molecular diagnostic assays is an important capability for clinical pathologists, basic researchers, pharmaceutical developers, and public healthcare experts. Laboratory molecular diagnostic testing is used to develop precision medicines for infectious diseases and for initial diagnosis of infectious diseases. As many infectious diseases have similar symptoms, it is necessary to determine the exact infectious disease for proper treatment, which is done using laboratory molecular diagnostics testing. To monitor the therapy used for treating infectious diseases, molecular diagnostic techniques are used to study the effect of the medicine. Point-of-care testing applications include detecting single, two, or more pathogens, evaluating emerging novel infections, surveillance and early detection of biothreat agents and diseases-related biomarkers, and antimicrobial resistance profiling. According to an article published in the National Library of Medicine, it is estimated that ~10 million people yearly die due to antimicrobial resistance (AMR). In AMR, certain bacteria develop resistance to antibiotics that are given to treat bacterial infections. To detect AMR, WHO has planned to emphasize effective, fast, and low-cost diagnostic technologies for guiding the appropriate use of antibiotics in human and animal medicine. This plan is proposed to improve the accuracy and speed of diagnosis of AMR using POC testing techniques. Increasing focus on developing and using POC testing tools for accurate diagnosis of AMR is fueling the market for the segment growth. Multiplex POC testing is used in critical care to detect two or more pathogens. In critical care, multiplex POC tests provide higher accuracy for actionable decision making, due to which the patient can receive pathogen-specific treatment. ID NOW, cobas Liat influenza A/B/RSV assay, and GeneXpert Xpress Flu/RSV are the three products clinically approved and available for detecting pathogens. The availability of multiplex POC testing for detecting pathogens is fuelling the market for the segment.
Molecular Diagnostic for Infectious Diseases Market Size and Forecast (2020 - 2030), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: Type (Point-of-Care Testing and Laboratory Testing), End User [Point-of-Care Testing (Human Testing and Vet Testing) and Laboratory Testing (Human Testing and Vet Testing)], Application [Point-of-Care Testing (Detection of Single Pathogen, Detection of Two or More Pathogens, Evaluation of Emerging Novel Infections, Surveillance and Early Detection of Biothreat Agents and Diseases-Related Biomarker, and Antimicrobial Resistance Profiling) and Laboratory Testing (Patient stratification, Drug Regimen Selection, Toxicity Avoidance, Therapeutic Monitoring, and Detection of Predisposition to Disease)], Disease Type [Point-of-Care Testing (Sepsis, Prosthetic Joint Infection (PJI), Endocarditis, STDs, Mononucleosis, Group A Streptococcus (GAS), and Others) and Laboratory Testing (Sepsis, Prosthetic Joint Infection (PJI), Endocarditis, STDs, Chlamydia, Gastrointestinal Infection, Tuberculosis, H1N1 Virus, and Others)], Infection Type [Point-of-Care Testing (Bacteria, Viral, Fungi, and Others) and Laboratory Testing (Bacteria, Viral, Fungi, and Others)], and Geography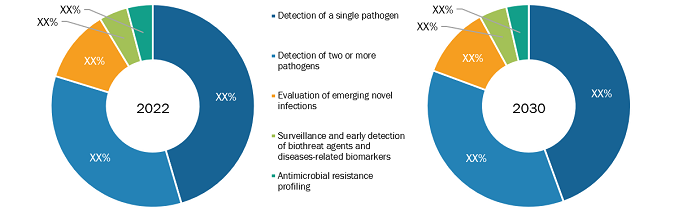
Molecular Diagnostic for Infectious Diseases Market 2030
Download Free Sample
Based on disease type, the molecular diagnostic for infectious diseases market is bifurcated into point-of-care testing and laboratory testing. Point-of-care testing is further segmented into detection of a sepsis, prosthetic joint infection, endocarditis, STDs, mononucleosis, group A streptococcus (GAS), and others. Laboratory testing is further segmented into sepsis, prosthetic joint infection, endocarditis, STDs, chlamydia, gastrointestinal infection, tuberculosis, H1N1 virus, and others. According to an article published in eHealth, India contributes ~27% of TB cases globally. As per the same source, 350–500 million people suffer from TB, and ~2.6 million people develop TB annually in India. To increase the diagnosis rate in India, SRL Diagnostics (an Indian company) launched an advanced genomic test for tuberculosis requiring whole genome sequencing. The increasing prevalence of STIs such as chlamydia, gonorrhea, vaginalis, and genitalium also stimulates the demand for products that can diagnose these STIs. In May 2022, Abbott received FDA clearance for Alinity m STI Assay. This technology uses PCR technology, which is highly sensitive to the abovementioned STIs. Thus, the growing product launches for diagnosing infectious diseases require laboratory testing. The high prevalence of HIV triggers the demand for point-of-care diagnostics. According to the UNAIDS, 1.5 million new HIV cases were registered globally in 2022. Due to the increase in HIV cases, United Nations International Children's Emergency Fund (UNICEF) focuses on procuring and supplying POC HIV viral load testing and early infant diagnosis to support pregnant women, infants, mothers, adolescents, and children. This initiative by UNICEF fuels the demand for point-of-care testing to diagnose HIV and STDs globally. The market is further driven by strategic initiatives such as product launches, mergers and acquisitions, and research and development activities by start-ups to develop POC testing for infectious diseases. In May 2023, Sensible Diagnostics, a US-based start-up, developed a POC-PCR system with low-cost test cartridges to perform infectious disease tests in 10 minutes.
Region Analysis – Molecular Diagnostic for Infectious Diseases Market
Based on geography, the molecular diagnostic for infectious diseases market is segmented into North America, Europe, Asia Pacific, the Middle East & Africa, and South & Central America. North America is the largest contributor in 2022 and Asia Pacific is expected to be the fastest-growing market in the coming years. The molecular diagnostics for infectious diseases market in Asia Pacific is subsegmented into Malaysia, Thailand, India, Singapore, the Philippines, Vietnam, Indonesia, China, Japan, Australia, South Korea, and the Rest of Asia Pacific. Rising geriatric population; increasing infectious diseases; growing research activities and technological advancements; and surging number of startups, biotechnology, and biopharmaceutical companies are the factors favoring the market progress in this region. Moreover, the presence of associations or organizations enhancing the quality of care in infectious disease management contributes to the molecular diagnostics for infectious disease market growth. According to a blog published by ChinaTown Online, ~1 in 7 people from the age range of 15–49 have been infected with STDs in China. Chlamydia, gonorrhea, genital warts, and syphilis are the most common STDs. According to the WHO, ~370 million new cases of trichomoniasis, Neisseria gonorrhea (NG), Chlamydia trachomatis (CT), and syphilis infections, among others, were detected in China in 2020. ~35% of the new infections registered were CT—one of the world’s most commonly reported sexually transmitted infections (STIs). According to the WHO, by the 27th week of 2023, ~4,250 people tested positive for influenza and nearly 23,300 specimens were processed for detection of influenza virus. This created a demand for molecular diagnostic products and services in Malaysia. Data published in the Korean Journal of Internal Medicine mentions that the incidence of TB in South Korea was 39 cases per 100,000 people in 2020. Moreover, the proportion of newly diagnosed cases of TB in elderly patients aged over 65 years has increased considerably from 27.5% (in 2008) to 48.5% (in 2020). According to the World Health Organization, Indonesia recorded 200 cases of human infection with the avian influenza A (H5N1) virus in January 2020. Of these, 168 cases were fatal and resulted in an 84% mortality rate (168/200), which is much higher than the global fatality rate of 53%. Molecular diagnostic tools such as polymerase chain reaction (PCR), sequencing, hybridization, and microarray are employed to detect these infectious diseases as they surface as rapid and sensitive approaches for screening, detection, and monitoring.
F.Hoffmann-La Roche Ltd, Abbott Laboratories, Thermo Fisher Scientific Inc, bioMerieux SA, Danaher Corp, Hologic Inc, Siemens Healthineers AG, Bruker Corp, Molzym GmbH & Co KG, and DiaSorin SpA are among the leading companies operating in the molecular diagnostic for infectious diseases market.
The report segments the molecular diagnostic for infectious diseases market as follows:
The molecular diagnostic for infectious diseases market is divided on the basis of type, disease type, infection type, application, and end user. Based on type, the market is classified into point-of-care testing and laboratory testing. The molecular diagnostic for infectious diseases market, by end user, is bifurcated into point-of-care testing and laboratory testing. Point-of-care testing is further bifurcated into human testing and vet testing. Similarly, laboratory testing is further bifurcated into human testing and vet testing. The molecular diagnostic for infectious diseases market, by application, is bifurcated into point-of-care testing and laboratory testing. Point-of-care testing is further segmented into detection of a single pathogen, detection of two or more pathogens, evaluation of emerging novel infections, surveillance and early detection of biothreat agents and diseases-related biomarkers, and antimicrobial resistance profiling. Laboratory testing is further segmented into patient stratification, drug regimen selection, toxicity avoidance, therapeutic monitoring, and detection of predisposition to disease. The molecular diagnostic for infectious diseases market, by disease type, is bifurcated into point-of-care testing and laboratory testing. Point-of-care testing is further segmented into detection of a sepsis, prosthetic joint infection, endocarditis, STDs, mononucleosis, group A streptococcus, and others. Laboratory testing is further segmented into sepsis, prosthetic joint infection, endocarditis, STDs, chlamydia, gastrointestinal infection, tuberculosis, H1N1 virus, and others. The molecular diagnostic for infectious diseases market, by infection type, is bifurcated into point-of-care testing and laboratory testing. Point-of-care testing is further segmented into bacteria, viral, fungi, and others. Laboratory testing is further segmented into bacteria, viral, fungi, and others. Based on geography, the market is segmented into North America (the US, Canada, and Mexico), Europe (the UK, Germany, France, Italy, Spain, Luxembourg, Austria, Belgium, the Netherlands, and Rest of Europe), Asia Pacific (China, Japan, India, South Korea, Indonesia, Malaysia, and the Rest of Asia Pacific), the Middle East & Africa (the UAE, Saudi Arabia, South Africa, and the Rest of Middle East & Africa), and South & Central America (Brazil and the Rest of South & Central America).
Contact Us
Phone: +1-646-491-9876
Email Id: sales@theinsightpartners.com