Viral Vectors Segment by Product Dominated the Viral Vector and Plasmid DNA Manufacturing Market During 2019–2027
According to our new research study on "Viral Vector and Plasmid DNA Manufacturing Market Forecast to 2027 – COVID-19 Impact and Global Analysis – by Product and Application," the viral vector and plasmid DNA manufacturing market size was valued at US$ 459.4 million in 2019 and is expected to reach US$ 2,247.7 million by 2027; it is estimated to record a CAGR of 22.1% from 2020–2027. The report highlights the trends and drivers prevailing in the market.
Viral Vector and Plasmid DNA Manufacturing Market, by Region, 2019 (%)
Viral Vector and Plasmid DNA Manufacturing Market Forecast to 2027 - COVID-19 Impact and Global Analysis by Product (Viral Vectors and Non-viral Vectors) and Application (Cancer, Inherited Disorders, Viral Infections, and Others)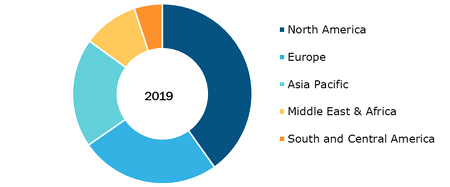
Viral Vector and Plasmid DNA Manufacturing Market Share 2027
Download Free Sample
Source: The Insight Partners Analysis
Based on the product, the viral vector and plasmid DNA manufacturing market is bifurcated into viral vectors and non-viral vectors. The viral vectors segment held the larger market share in 2019, however, the non-viral vectors segment is anticipated to register the higher CAGR in the upcoming years. Viral vectors are the devices that molecular biologists typically use to introduce genetic material into cells. This process can be carried out either in vivo or in vitro. Viruses have evolved specialized molecular mechanisms to efficiently transport their genomes inside the cells they infect. Viral vectors are used in basic research, gene therapy, and vaccine preparation. Viral vectors can be of different types such as retroviruses, lentiviruses, adenoviruses, adeno-associated viruses, and hybrids. Additionally, effective gene delivery, high transfection efficiency, and stable gene expression have made viral vectors a preferred choice for gene transfer. The rising preference for viral vectors in gene transfer is evident by the increase in number of clinical trials on viral vector-mediated gene therapy and rising in number of initiatives taken by market players. For instance, in 2019, around 372 clinical trials were registered that involved vector-mediated gene therapy production. Moreover, In October 2019, GE Healthcare Life Sciences has launched Flex Factory single-use biomanufacturing platform-based KUBio box, which is fully-integrated, and flexible biomanufacturing environment to affectively address the aforementioned challenge and speed-up the development of viral vector-based gene therapies. Thus, broad application of viral vectors, increasing registration of clinical trials, and rising in strategic initiative by the companies is likely to boost the viral vector in Viral Vector and Plasmid DNA manufacturing market growth.
Based on application the viral vector and plasmid DNA manufacturing market is categorized into cancer, inherited disorders, viral infections, and others. Based on geography, the viral vector and plasmid DNA manufacturing market is segmented into North America (the US, Canada, and Mexico), Europe (the UK, Germany, France, Italy, the Netherlands, and the Rest of Europe), Asia Pacific (China, Japan, India, Australia, Thailand, and the Rest of Asia Pacific), the Middle East & Africa (the UAE, Saudi Arabia, South Africa, and the Rest of Middle East & Africa), and South & Central America (Brazil, Argentina, and the Rest of South & Central America).
Impact of COVID-19 Pandemic on Viral Vector and Plasmid DNA Manufacturing Market
The outbreak of COVID-19 is expected to have positive effects on the production of vector and recombinant DNA. Viral vector vaccines also include COVID-19 vaccines that are introduced in several candidates as it entered clinical trials. For commercial use of such vaccines, it needs to be approved by the regulatory bodies to ensure safety and efficacy of such vaccines. There are various viral vector vaccines are approved for commercial use and some are under clinical trials. In order to assess the protection effectiveness of the vaccine against COVID-19 in preliminary studies, scientists created specific animal models such as human ACE2nHACE2), which express Tg mice, hamsters, and nonhuman primates. The Oxford-AstraZeneca COVID-19 viral vector-based vaccine has received approval from different regulatory bodies for human use. This vaccine was first developed in November 2020 and since then commercially produced in large quantities for human vaccination. Based on nonreplicating viruses, COVID-19 vaccines are being developed globally to deal with pandemic situation. These vaccines will have similar immune response as before, i.e., include antibodies produced in B cells and T cells targeting and destroying infected cells within the body that provides lifelong immunity. The increase in research and investments in this area is expected to benefit the development of vaccines. Thus, the overall COVID-19 impact on the viral vector and plasmid DNA manufacturing market growth was positive and the market is expected to flourish in the near future.
Contact Us
Phone: +1-646-491-9876
Email Id: sales@theinsightpartners.com