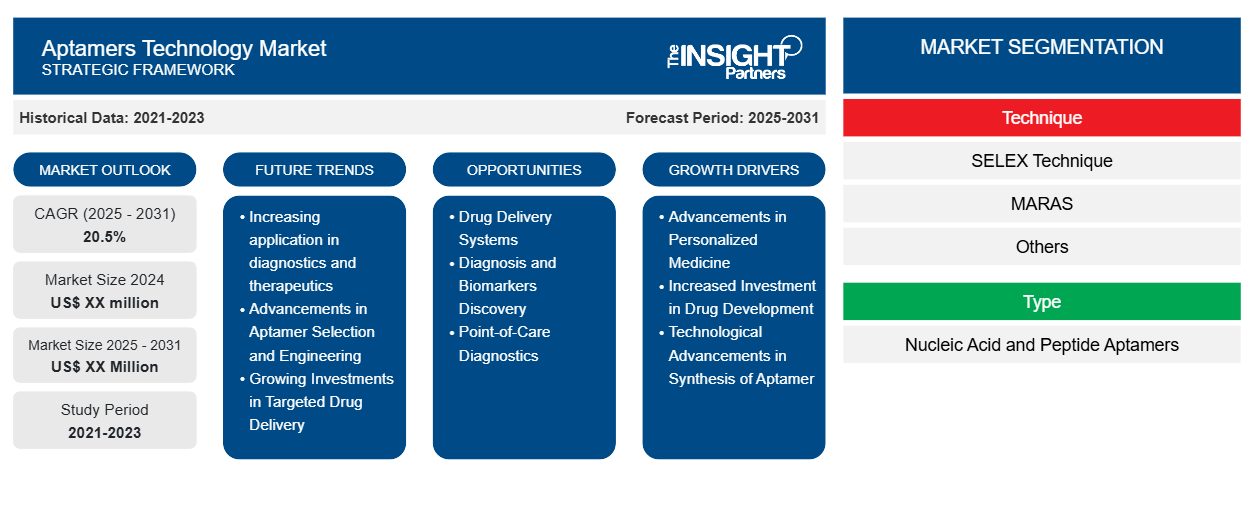
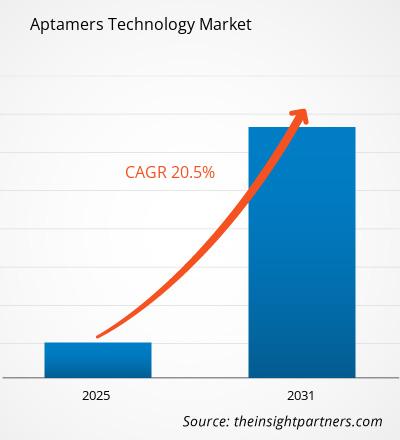
The Aptamers Technology Market is expected to register a CAGR of 20.5% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The report is segmented by Technique (SELEX Technique, MARAS, and Others), By Type (Nucleic Acid and Peptide Aptamers), Application (Diagnostics, Therapeutics, Research and Development), by End User (Biotechnology and Pharmaceutical Companies, Academic and Research Institutions, Contract Research Organization). The global analysis is further broken-down at regional level and major countries. The report offers the value in USD for the above analysis and segments
Purpose of the Report
The report Aptamers Technology Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
Aptamers Technology Market Segmentation
Technique
- SELEX Technique
- MARAS
- Others
Type
- Nucleic Acid and Peptide Aptamers
Application
- Diagnostics
- Therapeutics
- Research and Development
End User
- Biotechnology and Pharmaceutical Companies
- Academic and Research Institutions
- Contract Research Organization
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Aptamers Technology Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Aptamers Technology Market Growth Drivers
- Advancements in Personalized Medicine: Interest in aptamers, particularly through high specificity and low toxicity in targeted treatment, increased as the demand for personalized therapies grew. This also presents a molecular structure of an aptamer to which certain biomolecules may attach themselves, suitable for precise applications in fields like oncology and genetic disorder cases, thus making its applications viable for clinical and research practices.
- Increased Investment in Drug Development: Greater investments have now been made in drugs research in response to the current inflated prices and complexity of standard pharmaceutical development. This would open many gates toward alternative therapies; more on that later, like the interesting properties and behaviors of aptamers as nucleic-acid-based molecules, suggesting how new diagnostics and therapeutics might be developed by locking specific proteins or cells targeted upon that site.
- Technological Advancements in Synthesis of Aptamer: The technological developments of SELEX have improved efficiency and scalability in the process of aptamer synthesis. Developments that increase affinities and specificities of aptamers develop these in therapeutic, diagnostics, and biosensing applications; these developments will contribute to driving growth in the market.
Aptamers Technology Market Future Trends
- Increasing application in diagnostics and therapeutics: The growth in diagnostics and therapeutics applications is driving the market. Aptamers, possessing high affinity and specificity towards target molecules, find utility in early detection of diseases and as therapeutic agents for diseases like cancer. This dual application of aptamers in medical research and clinical treatments extends the usage of aptamers and fuels the demand for the market.
- Advancements in Aptamer Selection and Engineering: Advances in aptamer selection and engineering by technologies have significantly improved the specificity and stability of aptamers. Development of techniques such as SELEX is further being developed for efficient development of aptamers. All these developments make aptamers more effective, thereby raising their applications in research, diagnostics, and drug development.
- Growing Investments in Targeted Drug Delivery: One of the trends in aptamers technology market includes targeted drug delivery, on which focus has been increasing on this particular aspect. Aptamers are currently under research, so the direct delivery of drugs can directly target specific cells or tissues for the improved therapy outcomes and reduced adverse effects. Aptamers are turning out to be an extremely promising tool, mainly with respect to precise drug delivery systems.
Aptamers Technology Market Opportunities
- Drug Delivery Systems: Aptamers are beginning to make their way into actual application as a drug delivery system on account of their target specificity very precise cell or tissue targeting. Thus therapeutic agents linked with them may be marketed by companies offering much efficacious but less invasive therapies. Such a course would open new horizons in nanomedicine for controlled, site-specific delivery and effective but safer drug administration.
- Diagnosis and Biomarkers Discovery: The new aptamer and application opportunities in diagnostics-these may become sole high-specificity biomarkers for early detection of disease. This smaller form of nucleic acid is relatively stable, and its efficiency is sufficient for using it in point-of-care diagnosis or biosensors, thus promoting early disease detection. This will thus speak well for the market feeling about detection of traces of biomarkers improving the conditions of many.
- Point-of-Care Diagnostics: Aptamers stand to offer a considerable new opportunity in the point-of-care diagnostics market because of their target specificity and affinity for molecules. Used in point-of-care portable diagnostic devices, they can provide fast and direct on-site disease detection, such as infectious diseases and cancer. They are also very cost-effective and would easily integrate into diagnostic platforms, making them ideal candidates for such emerging healthcare markets.
Aptamers Technology Market Regional Insights
The regional trends and factors influencing the Aptamers Technology Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Aptamers Technology Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Aptamers Technology Market
Aptamers Technology Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ XX million |
| Market Size by 2031 | US$ XX Million |
| Global CAGR (2025 - 2031) | 20.5% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Technique
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Aptamers Technology Market Players Density: Understanding Its Impact on Business Dynamics
The Aptamers Technology Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Aptamers Technology Market are:
- SomaLogic
- Aptamer Group
- Aptadel Therapeutics
- Base Pair Biotechnologies
- Noxxon Pharma
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Aptamers Technology Market top key players overview
Key Selling Points
- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the Aptamers Technology Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the Aptamers Technology Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Technique, Type, Application, End User, and Geography

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
Some of the customization options available based on the request are an additional 3-5 company profiles and country-specific analysis of 3-5 countries of your choice. Customizations are to be requested/discussed before making final order confirmation# as our team would review the same and check the feasibility
The report can be delivered in PDF/PPT format; we can also share excel dataset based on the request
Rising demand for targeted therapeutics and increased investment in biotechnology and drug development are the major factors driving the aptamers technology market.
Development of aptamers for personalized medicine is likely to remain a key trend in the market.
The Aptamers Technology Market is estimated to witness a CAGR of 20.5% from 2023 to 2031
Trends and growth analysis reports related to Life Sciences : READ MORE..
- SomaLogic
- Aptamer Group
- Aptadel Therapeutics
- Base Pair Biotechnologies
- Noxxon Pharma
- Vivonics Inc.
- Aptagen, LLC
- TriLink Biotechnologies
- Altermune LLC
- AM Biotechnologies
 Get Free Sample For
Get Free Sample For