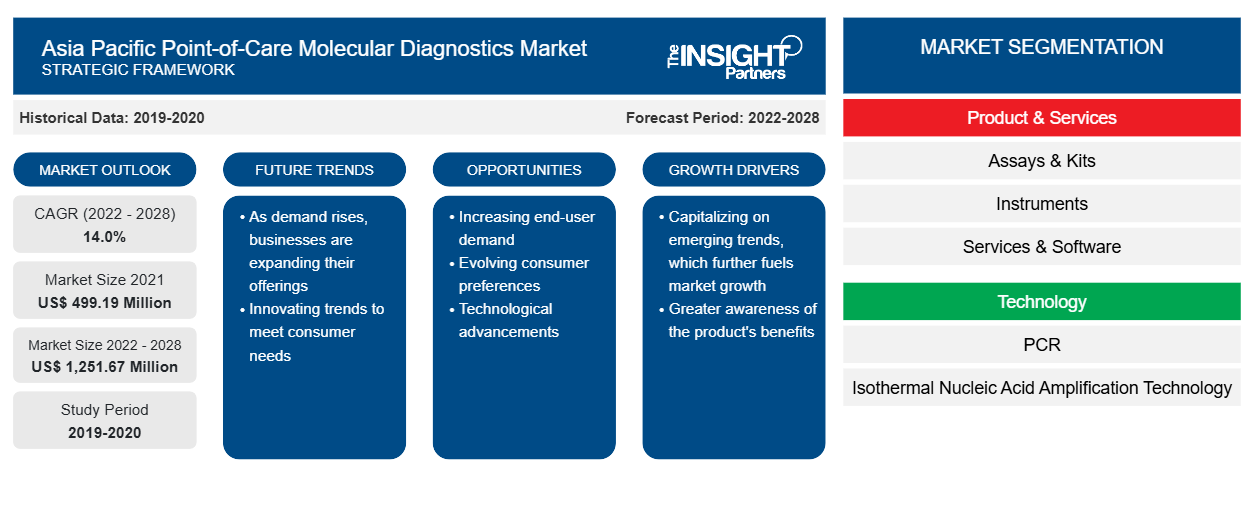
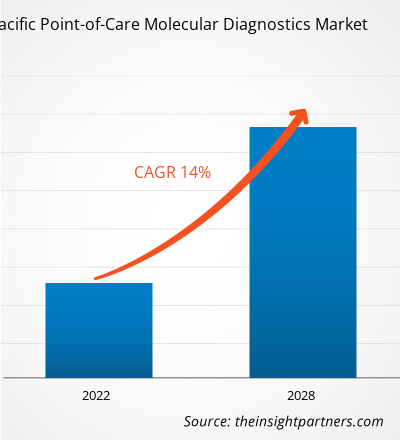
The Asia Pacific point-of-care molecular diagnostics market is projected to reach US$ 1,251.67 million by 2028 from US$ 499.19 million in 2021; it is expected to grow at a CAGR of 14.0% from 2022 to 2028.
The growth of the market is attributed to the growing demand for specific viral detection methods that consume less time for timely infection control and rising incidence of infectious diseases. However, the pricing pressures owing to reimbursement cuts restrain the market growth. Point-of-care molecular diagnostics comprises portable devices and assays & kits to detect and diagnose diseases from human samples, such as throat swab, blood, serum, and stool. Molecular diagnostics are shifting from centralized laboratories to decentralized point-of-care molecular testing, due to its simplicity, convenience, rapid turnaround time, and potential to improve patient outcomes. Owing to these advantages, it can be applied for diagnosis in low-resource or remote areas.
The continuous spread of the disease has led to an elevated demand for advanced diagnostic solutions in Asia Pacific, thus boosting the adoption of point-of-care molecular diagnostic kits. Moreover, uninterrupted investments and business activities by industry players are also boosting the point-of-care molecular diagnostic market growth.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONAsia Pacific Point-of-Care Molecular Diagnostics Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Market Insights
Rising Incidence of Infectious Diseases Fuels Asia Pacific Point-of-Care Molecular Diagnostics Market Growth
The rising spread of infectious diseases increases the level and rate of testing. Moving molecular diagnostic testing for infectious diseases from laboratories to the point-of-care settings has the potential to revolutionize the rate and amount of testing to be performed. Molecular diagnostics is a more sensitive product and services that allows the detection of smaller concentrations of infectious pathogens, allowing diseases detection earlier than previously allowed. Point-of-care molecular diagnostics offers the potential to minimize the time required to get an actionable result and promote early infection detection, appropriate infection control measures, and enrollment into therapy clinical trials. The rising prevalence of influenza A/B, respiratory syncytial virus (RSV), and hospital-acquired infections (HAIs) boosts the demand for point-of-care molecular testing. Influenza and respiratory syncytial virus (RSV) point-of-care testing can improve patient treatment and infection control. Recently, Asia Pacific point-of-care molecular diagnostics played a crucial role in detecting the COVID-19 infection. For COVID-19 infection detection, RT-PCR-based diagnostic tests are time-consuming, expensive, and require advanced equipment and specialized personnel. The high cost of diagnosis and the scarcity of test kits made it difficult to monitor the community transmission. As a result, rapid, affordable, and effective approaches for detecting COVID-19 viral infection in people were needed urgently. The easy and effective point-of-care molecular diagnostic devices allow on-site testing, which aids in the prevention of infection and control of its spread. Therefore, the rising demand for rapid and effective point-of-care molecular kits to detect infectious diseases drives the market growth.
Product & Services -Based Insights
Based on product & services, the Asia Pacific point-of-care molecular diagnostics market is segmented into assays & kits, instruments, and services & software. The assays & kits segment holds the largest share of the market and is anticipated to register the highest CAGR during 2022–2028. The assays & kits help in the early diagnosis of respiratory tract infections and women’s health and sexual health conditions. Also, they are used in various applications such as studying disease pathways, screening for potential drug candidates, and evaluating biopharmaceutical production processes.
Asia Pacific Point-of-Care Molecular Diagnostics Market, by Product & Services – 2021 and 2028
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Technology-Based Insights
Based on technology, the Asia Pacific point-of-care molecular diagnostics market is segmented into PCR, isothermal nucleic acid amplification technology (INAAT), and other technologies. The PCR segment holds the largest share of the market, whereas the isothermal nucleic acid amplification technology (INAAT)segment is anticipated to register the highest CAGR in the market during the forecast period.
Application-Based Insights
Based on application, the Asia Pacific point-of-care molecular diagnostics market is segmented into infectious diseases, oncology, hematology, prenatal testing, endocrinology, and other applications. The infectious diseases segment holds the largest share of the market. However, the oncology segment is anticipated to register the highest CAGR in the market during the forecast period.
End User-Based Insights
Based on end user, the Asia Pacific point-of-care molecular diagnostics market is segmented into hospitals & clinics, diagnostic laboratories, research & academic institutes, and others. The diagnostic laboratories segment holds the largest market share and is estimated to register the highest CAGR during the forecast period. The market growth for this segment is attributed to the innovations in Asia Pacific point-of-care molecular diagnostics.
Companies operating in the Asia Pacific point-of-care molecular diagnostics market are adopting the product innovations strategy to meet the evolving customer demands across the world, which also permits them to maintain their brand name in the global market.
The Asia Pacific point-of-care molecular diagnostics market is segmented on the basis of product & services, technology, application, and end user. Based on product & services, the market is segmented into assays & kits, instruments, and services & software. Based on technology, the market is segmented into PCR, isothermal nucleic acid amplification technology (INAAT), and other technologies. Based on application, the Asia Pacific point-of-care molecular diagnostics market is segmented into infectious diseases, oncology, hematology, prenatal testing, endocrinology, and other applications. By end user, the market is segmented into hospitals & clinics, diagnostic laboratories, research & academic institutes, and others. Based on countries, the Asia Pacific point-of-care molecular diagnostics market is segmented into China, Japan, India, Australia, South Korea, Thailand, and Rest of Asia Pacific. bioMérieux SA; F. Hoffmann-La Roche Ltd.; Danaher Corporation; Enzo Biochem, Inc.; Abbott; binx health Inc.; Meridian BioScience, Inc.; Biocartis, Quidel Corporation, and Bio-Rad Laboratories, Inc. are a few leading companies operating in the Asia Pacific point-of-care molecular diagnostics market.
Report ScopeAsia Pacific Point-of-Care Molecular Diagnostics Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 499.19 Million |
| Market Size by 2028 | US$ 1,251.67 Million |
| CAGR (2022 - 2028) | 14.0% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Product & Services
|
| Regions and Countries Covered |
Asia-Pacific
|
| Market leaders and key company profiles |
|
Asia Pacific Point-of-Care Molecular Diagnostics Market Players Density: Understanding Its Impact on Business Dynamics
The Asia Pacific Point-of-Care Molecular Diagnostics Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Asia Pacific Point-of-Care Molecular Diagnostics Market top key players overview
Frequently Asked Questions
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends






















 Get Free Sample For
Get Free Sample For