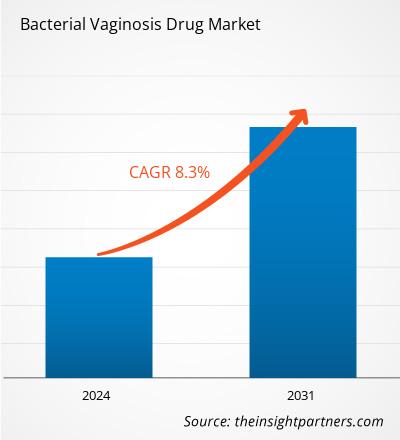
The bacterial vaginosis drug market size is projected to reach US$ 3.82 billion by 2031 from US$ 2.15 billion in 2024. The market is expected to register a CAGR of 8.3% during 2024–2031. Rising inclination toward microbiome therapies and probiotics are likely to bring in new market trends during the forecast period.
Bacterial Vaginosis Drug Market Analysis
Bacterial vaginosis (BV) is a common condition experienced by women of childbearing age, which is generally caused by changes in the normal bacterial flora of the vagina. BV is characterized by a decrease in Lactobacillus species and an increase in anaerobic bacteria such as Ureaplasma urealyticum, Gardnerella vaginalis, Mobiluncus species, Mycoplasma hominis, and Prevotella species. Activities such as unprotected sexual intercourse and frequent douching further increase the risk of BV. Common symptoms of the condition include abnormal vaginal discharge, odor, and itching. According to the European Journal of Obstetrics & Gynecology and Reproductive Biology article published in ScienceDirect Journal in 2023, the global prevalence of BV ranges from 23% to 29%, and in pregnant women, it ranges from 11% to 49%. According to a report by Medscape in 2024, approximately one-third of adult women, i.e., ~22 million, in the US are affected by BV every year, and 10 million women visit clinics for vaginal discharge. BV poses risks such as preterm labor, miscarriage, and increased susceptibility to HIV and other sexually transmitted infections. The increasing incidence of BV, coupled with potential complications, encourages women to seek medical attention and pharmaceutical interventions, creating significant demand for effective drugs. The high chances of recurrence of BV lead to the development of treatment regimens to prevent relapses. As healthcare access improves globally, especially in emerging markets, a larger number of women are now seeking treatment for BV, driving the growth of the bacterial vaginosis drug market
Bacterial Vaginosis Drug Market Overview
The bacterial vaginosis drug market is rapidly expanding due to the rising prevalence of the disease and the increased focus on developing novel and targeted therapies. Prominent players operating in the market are focusing on innovation and collaboration for enhanced product availability. However, the high recurrence rate and treatment adherence challenges hinder the bacterial vaginosis drug market growth.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Bacterial Vaginosis Drug Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Bacterial Vaginosis Drug Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Bacterial Vaginosis Drug Market Drivers and Opportunities
Increased Focus on Developing Novel and Targeted Therapies Fuels Market Growth
Pharmaceutical companies and research institutions are continuously focusing on the development of innovative treatments to target the root causes of BV, along with enhancing the safety and efficacy of existing drugs. Former BV treatments include antibiotics such as metronidazole and clindamycin, which work by eliminating the overgrowth of harmful bacteria in the vagina. However, a high recurrence rate and potential side effects such as gastrointestinal issues and yeast infections are among the typical limitations associated with these treatments. This drawback has led to the development of advanced and more targeted therapies, delivering improved patient outcomes with minimum adverse effects. Prominent improvements in BV treatment include the development of single-dose therapies such as secnidazole, which is an oral antibiotic that has demonstrated efficacy similar to standard treatments but with a more convenient administration schedule and fewer side effects. Secnidazole has gained popularity because of its high cure rate and its ability to reduce the risk of BV recurrence compared to longer, multidose antibiotic regimens. This shift toward more patient-friendly treatment options is contributing to the growth of the BV drug market, as patients are more likely to follow simple regimens. Therefore, the increased availability of novel drugs (such as secnidazole) and innovative drug delivery systems (including vaginal gels and inserts) that improve treatment adherence and minimize side effects are contributing to the bacterial vaginosis drug market growth.
Strategic Initiatives by Market Players to Create Lucrative Opportunities in Market
Companies operating in the bacterial vaginosis drug market constantly focus on strategic developments such as product approvals, collaborations, funding, agreements, and new product launches, which help them improve their sales, increase their geographic reach, and reinforce their capacities to cater to a greater than existing customer base. A few strategic initiatives by key players operating in the bacterial vaginosis drug market are mentioned below.
- In November 2024, Freya Biosciences received US$ 10.4 million in strategic investment from the Bill & Melinda Gates Foundation, with US$ 1.4 million in additional financing from the current investor—Export and Investment Fund of Denmark (EIFO). The company plans to use these funds to develop microbial immunotherapies for the treatment of BV to address the risk of preterm birth along with other indications potentially affecting maternal and newborn health.
- In March 2022, Organon and Daré Bioscience, Inc. entered into an agreement whereby Organon will license global rights to XACIATO (clindamycin phosphate vaginal gel, 2%). With this, XACIATO received both Qualified Infectious Disease Product (QIDP) and Fast Track designations from the US Food and Drug Administration (FDA) for the treatment of bacterial vaginosis.
- In February 2022, Lupin Pharmaceuticals Inc. received FDA approval for a supplemental New Drug Application (sNDA) to increase the use of SOLOSEC (secnidazole) for the treatment of BV among female patients aged 12 or older; the approval also entailed the treatment of trichomoniasis among patients from the said age group.
- In December 2021, the FDA approved XACIATO (clindamycin phosphate vaginal gel, 2%) manufactured by Daré Bioscience, Inc. for the treatment of bacterial vaginosis in females of age 12 years or more.
- In February 2021, Rebiotix (Ferring) and MyBiotics collaborated to develop live microbiome-based therapeutics for applications in reproductive medicine and maternal health. This collaboration aims to create live microbiota-based biotherapeutics for bacterial vaginosis among women of childbearing age, which may be associated with the increased risk of miscarriage and complications in pregnancy and fertility.
Therefore, ongoing funding, product launches and approvals, and collaborations are expected to create ample opportunities for the growth of the bacterial vaginosis drug market in the coming years.
Bacterial Vaginosis Drug Market Report Segmentation Analysis
Key segments that contributed to the derivation of the bacterial vaginosis drug market analysis are type, route of administration, dosage form, and distribution channel.
- Based on type, the bacterial vaginosis drug market is segmented into prescription drugs and over-the-counter drugs. The prescription drugs segment held a larger share of the market in 2024.
- In terms of route of administration, the bacterial vaginosis drug market is categorized into oral, vaginal, and topical. The oral segment dominated the market in 2024.
- In terms of dosage form, the bacterial vaginosis drug market is categorized into pills, creams, gels, solutions/washes, and others. The pills segment dominated the market in 2024.
- In terms of distribution channel, the bacterial vaginosis drug market is categorized into hospital pharmacies, online pharmacies, and retail pharmacies. The hospital pharmacies segment dominated the market in 2024.
Bacterial Vaginosis Drug Market Share Analysis by Geography
The geographic scope of the bacterial vaginosis drug market report mainly focuses on five regions: North America, Asia Pacific, Europe, South & Central America, and the Middle East & Africa. In terms of revenue, North America dominated the market in 2024. It is estimated to dominate the global market during the forecast period. The US is the largest market for bacterial vaginosis drugs in the world. In the US, the bacterial vaginosis drug market is experiencing significant growth due to the increasing prevalence of BV, rising awareness about women's health, and ongoing efforts for the development of innovative treatments. BV is one of the most common vaginal infections in women, particularly affecting those of reproductive age. According to a report by Medscape in 2024, approximately one-third of adult women in the US, i.e., ~22 million females, are affected by BV annually, and 10 million women visit clinics for abnormal vaginal discharge. It is often linked to factors such as sexual activity, douching, and hormonal changes; nonetheless, there is uncertainty around the exact causes of BV. The primary therapeutic options available for treating BV include antibiotics approved by the US Food and Drug Administration (FDA), such as metronidazole and clindamycin. Metronidazole, often available in oral and topical forms, is considered the first-line treatment for BV. Clindamycin is also commonly available in both oral and topical formulations. Newer drugs and formulations, such as probiotics and phage therapy, are also emerging to help restore the natural vaginal flora, thereby reducing the recurrence of BV.
Government initiatives in the US play a key role in creating and spreading awareness about vaginosis in the population. The National Institutes of Health (NIH) funds research into the causes, treatment, and prevention of BV. The NIH's efforts have led to a deeper understanding of the microbiome and its role in causing BV, which is crucial for developing more targeted therapies. Centers for Disease Control and Prevention (CDC) provide guidelines and recommendations to improve the diagnosis and management of BV. These initiatives help standardize care and ensure that patients receive the most effective treatment. Additionally, the National Institute of Child Health and Human Development (NICHD, a federal agency) and NIH are dedicated to understanding BV and educating women about its causes and prevention. NICHD funds and conducts research on the risks associated with BV and methods for its prevention and treatment.
Bacterial Vaginosis Drug Market Regional Insights
The regional trends and factors influencing the Bacterial Vaginosis Drug Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Bacterial Vaginosis Drug Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Bacterial Vaginosis Drug Market
Bacterial Vaginosis Drug Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ 2.15 Billion |
| Market Size by 2031 | US$ 3.82 Billion |
| Global CAGR (2024 - 2031) | 8.3% |
| Historical Data | 2021-2023 |
| Forecast period | 2024-2031 |
| Segments Covered |
By Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Bacterial Vaginosis Drug Market Players Density: Understanding Its Impact on Business Dynamics
The Bacterial Vaginosis Drug Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Bacterial Vaginosis Drug Market are:
- Bayer AG
- Pfizer Inc
- Sanofi SA
- Starpharma Holdings Limited
- Viatris Inc.
- Glenmark Pharmaceuticals Ltd
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Bacterial Vaginosis Drug Market top key players overview
Bacterial Vaginosis Drug Market News and Recent Developments
The bacterial vaginosis drug market is evaluated by gathering qualitative and quantitative data post primary and secondary research, which includes important corporate publications, association data, and databases. A few of the key developments witnessed in the bacterial vaginosis drug market are listed below:
- VivaGel BV salesStarpharma inks partnership to distribute VivaGel BV product in MENA region and distribution agreement – MENA region. (Source: Starpharma Holdings Limited, Press Release, January 2024)
- Organon’s XACIATO (clindamycin phosphate) Vaginal Gel 2% was made available nationwide to treat bv in females aged 12 and older. (Source: Lupin, Press Release, January 2024)
- Glenmark Pharmaceuticals received ANDA approval for Metronidazole Vaginal Gel, 0.75%. (Source: Glenmark Pharmaceuticals Inc., Press Release, January 2022)
- Lupin announced sNDA approval by the FDA for SOLOSEC (secnidazole) to extend its use in adolescents for the treatment of both bacterial vaginosis and trichomoniasis in females. (Source: Lupin, Press Release, February 2022)
Bacterial Vaginosis Drug Market Report Coverage and Deliverables
The "Bacterial Vaginosis Drug Market Size and Forecast (2021–2031)" report provides a detailed analysis of the market covering below areas:
- Bacterial vaginosis drug market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Bacterial vaginosis drug market trends, as well as market dynamics such as drivers, restraints, and key opportunities
- Detailed PEST and SWOT analysis
- Bacterial vaginosis drug market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments for the bacterial vaginosis drug market
- Detailed company profiles
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
This text is related
to segments covered.

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
The market is expected to register a CAGR of 8.3% during 2024–2031.
The bacterial vaginosis drug market value is expected to reach US$ 3.82 billion by 2031.
Bayer AG, Pfizer Inc, Sanofi SA, Starpharma Holdings Limited, Viatris Inc, Glenmark Pharmaceuticals Ltd, Lupin Ltd, Sun Pharmaceutical Industries Ltd, Organon & Co, Duchesnay Inc., Canada are among the key players operating in the market.
Inclination toward microbiome therapies and probiotics are likely to emerge as new growth trends in the market in the coming years.
Rising prevalence of bacterial vaginosis, and the increased focus on developing novel and targeted therapies are among the most significant factors fueling the market growth.
North America dominated the market in 2023.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Bacterial Vaginosis Drug Market
- Bayer AG
- Pfizer Inc
- Sanofi SA
- Starpharma Holdings Limited
- Viatris Inc.
- Glenmark Pharmaceuticals Ltd
- Lupin Ltd
- Sun Pharmaceutical Industries Ltd
- Organon & Co
- Duchesnay Inc., Canada
 Get Free Sample For
Get Free Sample For