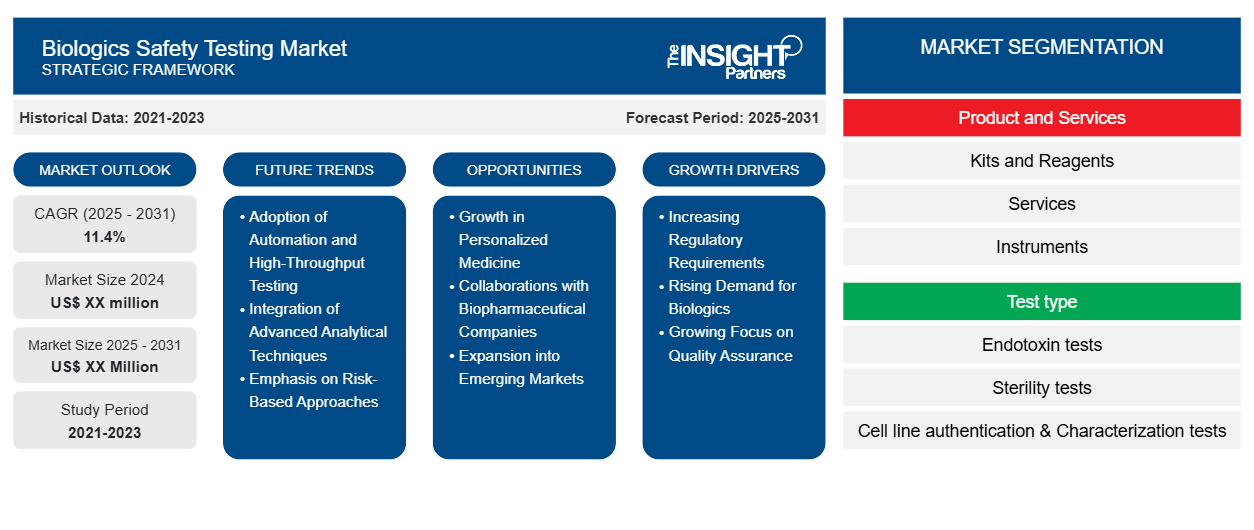
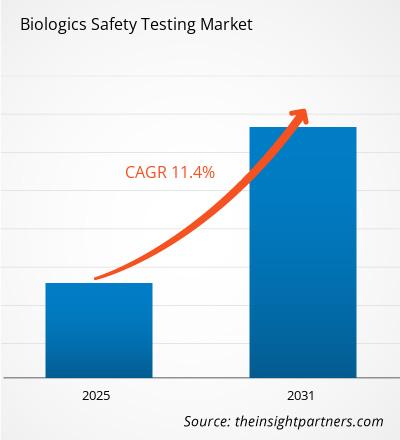
The Biologics Safety Testing Market is expected to register a CAGR of 11.4% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The report is segmented by Product and Services (Kits and Reagents, Services, and Instruments), Test type (Endotoxin tests, Sterility tests, Cell line authentication & Characterization tests, Cell line authentication, Bioburden tests, Residual host contaminant detection tests, Adventitious agent detection tests, and Others), Application (Vaccine development, Blood products testing, Cellular and gene therapy, Tissue & tissue-related products testing, and Stem cell research), End user (Pharmaceutical & Biotechnology Company, CROs & CDMOs, Academic & Research Centers)
Purpose of the Report
The report Biologics Safety Testing Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
Biologics Safety Testing Market Segmentation
Product and Services
- Kits and Reagents
- Services
- Instruments
Test type
- Endotoxin tests
- Sterility tests
- Cell line authentication & Characterization tests
- Cell line authentication
- Bioburden tests
- Residual host contaminant detection tests
- Adventitious agent detection tests
- Others
Application
- Vaccine development
- Blood products testing
- Cellular and gene therapy
- Tissue & tissue-related products testing
- Stem cell research
End user
- Pharmaceutical & Biotechnology Company
- CROs & CDMOs
- Academic & Research Centers
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Biologics Safety Testing Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Biologics Safety Testing Market Growth Drivers
- Increasing Regulatory Requirements: The growth in the regulatory requirements for biologics safety testing is one primary driver of market growth. For example, the FDA and EMA have developed and enforced other standards for the testing of such biologics and if exercised to the letter, will compel the organizations to improve their safety testing techniques. This ‘up-scaling’ is driven not only by patent expiries but also by additional requirements for testing resulting in new exploration of markets.
- Rising Demand for Biologics: The increasing effectiveness of biologics in treating chronic and complex illnesses contributes to their increased acceptance. This leads to growth in the biologics safety testing market. The expansion of the biopharma industry signifies that more and more biologics will be developed and put onto the shelves. As a result, this calls for effective safety evaluations of such products. This means that there is large capacity for production without necessarily incurring large costs due to the manufacturing of these tests and other services associated with safety of these therapies.
- Growing Focus on Quality Assurance: The rise in quality assurance concerns within the biopharmaceutical market enhances the market with regards to biologics safety testing. Manufacturer's growing interest toward stringent testing processes to avoid product recalls and adverse reaction risks fuels the demand in this market. It is more than just the rise in the market that has ulterior motives such as the provision of a reliable biologics safety testing that will concern patients as another factor contributing to the rising market requirements.
Biologics Safety Testing Market Future Trends
- Adoption of Automation and High-Throughput Testing: This upward trend in this market indicates the adoption of automation and high-throughput testing. These technologies make it easier to optimize the flow of such testing processes by allowing big samples to be analyzed in laboratories. High productivity, reduced errors, and improved reliability of safety assessments are associated with increased demands for results. Moreover, these reduce timelines associated with biologics development.
- Integration of Advanced Analytical Techniques: The face of biologics safety testing is changing through advanced technologies in analytical technologies. There is next-generation sequencing and mass spectrometry, among others. These novel technologies are capable of more precise detection and heightened sensitivity to impurities, contaminants, and genetic variations. Biopharmaceutical companies, along with regulatory shifts, will change with the times, delivering increased accuracy for safety tests and compliance - and thus the safe-guarding of patients.
- Emphasis on Risk-Based Approaches: Biologics safety testing is a trend that results from greater emphasis on the implementation of risk-based approaches. Rather than just merely applying traditional testing methods, more firms are now searching for and mitigating specific biologic-specific risks that could adversely affect their products. This approach actively allows organizations to use resources more effectively and ensure the best conditions for safety at various development points.
Biologics Safety Testing Market Opportunities
- Growth in Personalized Medicine: The biologics safety testing market has huge scope in the landscape of personalized medicine. Customized treatment to patients based on profiles builds up further demands for particular safety testing. Companies can leverage on this by offering customized testing solutions to the specific needs of safety testing personalized biologics. Thus, enhanced outcome for patients and increased market share are achieved.
- Collaborations with Biopharmaceutical Companies: Collaboration with biopharmaceutical companies presents an otherwise much-needed window for testing providers regarding the safety of biologics. In that respect, collaboration with them equips the testing laboratory with up-to-date developments concerning biologics to adjust their services accordingly. Indeed, collaboration perfects the development of testing methodology, efficiency levels, and review process as followed in respect to the current industry standards and guidelines on the safety review process.
- Expansion into Emerging Markets: Geographic expansion is one of the key growth drivers propelling biologics safety testing market growth. These geographies are constantly establishing their biopharmaceutical industries, and the need for safe testing services will only increase further. Companies that strategically start making their foray into these markets can capitalise on surging demand for biologics and their associated testing solutions, build out their global footprint, and contribute to better health care outcomes among underserved populations.
Biologics Safety Testing Market Regional Insights
The regional trends and factors influencing the Biologics Safety Testing Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Biologics Safety Testing Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
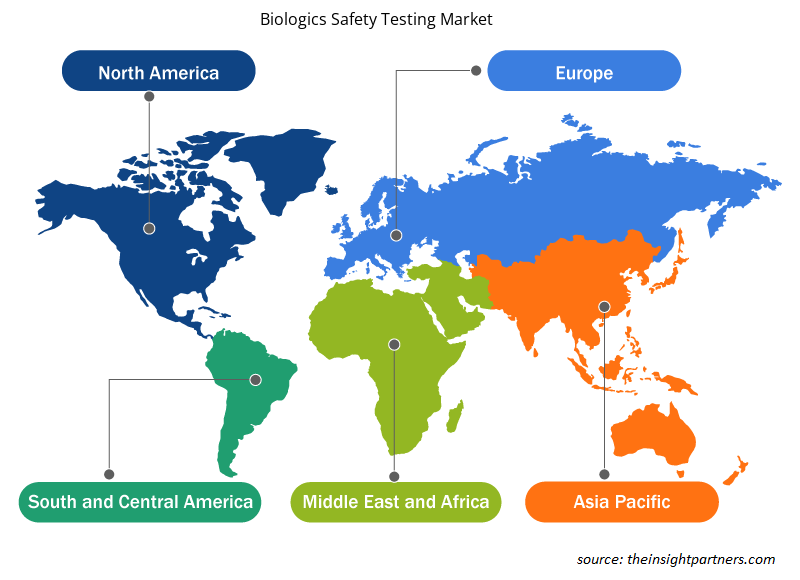
- Get the Regional Specific Data for Biologics Safety Testing Market
Biologics Safety Testing Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ XX million |
| Market Size by 2031 | US$ XX Million |
| Global CAGR (2025 - 2031) | 11.4% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Product and Services
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Biologics Safety Testing Market Players Density: Understanding Its Impact on Business Dynamics
The Biologics Safety Testing Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Biologics Safety Testing Market are:
- Avance Biosciences Inc.
- Charles River Laboratories International, Inc.
- Cytovance Biologics, Inc.
- Eurofins Scientific Se
- Lonza Group Ltd.
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Biologics Safety Testing Market top key players overview
Key Selling Points
- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the Biologics Safety Testing Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the Biologics Safety Testing Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
This text is related
to segments covered.

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
The biologics safety testing market is estimated to grow with a CAGR of 11.4% from 2023 to 2031.
Asia Pacific region is likely to witness fastest growth rate during the forecast period.
The biologics safety testing market majorly consists of the players such as Agilent Technologies, Inc.,Almac Group, and BioMérieux SA among others.
The market drivers include increasing regulatory requirements and rising demand for biologics are driving the biologics safety testing market
Adoption of automation and high-throughput testing are likely to remain the key trend during the forecast period
North America dominated the biologics safety testing market in 2023
Trends and growth analysis reports related to Life Sciences : READ MORE..
1. Avance Biosciences Inc.
2. Charles River Laboratories International, Inc.
3. Cytovance Biologics, Inc.
4. Eurofins Scientific Se
5. Lonza Group Ltd.
6. Merck KGaA
7. SGS S. A.
8. Wuxi Apptec
9. Sartorius AG
10. Toxikon Corporation
11. Thermo Fisher Scientific Inc.
12. Pace Analytical Services Inc.
 Get Free Sample For
Get Free Sample For