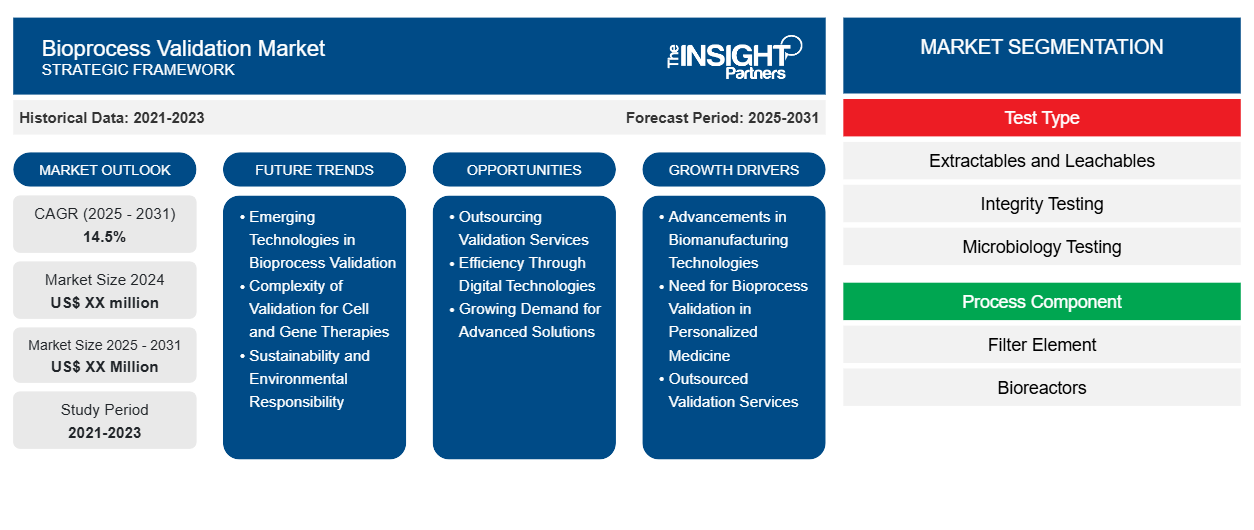
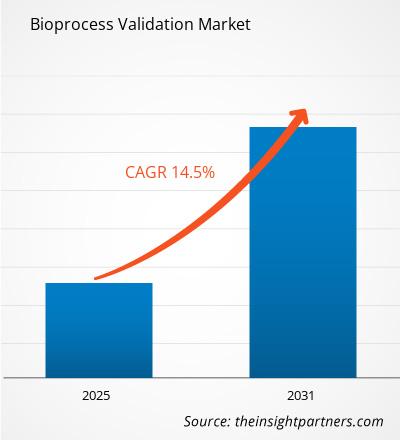
The Bioprocess Validation Market is expected to register a CAGR of 14.5% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The Bioprocess Validation market research report is segmented by test type into the following subsegments: Extractables and Leachables, Integrity Testing, and Microbiology Testing. The report further provides an analysis based on process component, i.e., Filter Element and Bioreactors. The market is also segmented by end-user, i.e., CDMO and Biotechnology & Pharmaceutical Companies. The market evaluation is presented in US$ for the above segmental analysis.
Purpose of the Report
The report Bioprocess Validation Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
Bioprocess Validation Market Segmentation
Test Type
- Extractables and Leachables
- Integrity Testing
- Microbiology Testing
Process Component
- Filter Element
- Bioreactors
End-User
- CDMO
- Biotechnology & Pharmaceutical Companies
Geography
- North America
- Europe
- Asia-Pacific
- South and Central America
- Middle East and Africa
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Bioprocess Validation Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Bioprocess Validation Market Growth Drivers
- Advancements in Biomanufacturing Technologies: Biomanufacturing technologies are advancing with single-use systems and continuous manufacturing, which necessitate comprehensive validation strategies for ongoing compliance and process optimization.
- Need for Bioprocess Validation in Personalized Medicine: The growing interest in personalized medicine and cell and gene therapies drives the demand for high-quality bioprocess validation to ensure effective production.
- Outsourced Validation Services: The outsourcing of validation services to specialized contract research organizations (CROs) and contract manufacturing organizations (CMOs) serves as a catalyst for the growth of the bioprocess validation market.
Bioprocess Validation Market Future Trends
- Emerging Technologies in Bioprocess Validation: Advanced technologies like artificial intelligence (AI), machine learning (ML), and digitalization will revolutionize validation processes through predictive analytics, real-time monitoring, and automated data analysis.
- Complexity of Validation for Cell and Gene Therapies: The increasing complexity and uniqueness of validation requirements driven by cell and gene therapies and personalized medicine will shape future bioprocess validation needs.
- Sustainability and Environmental Responsibility: Growing concerns about sustainability and environmental impact will drive the adoption of more environmentally friendly bioprocesses, which will require validation to meet regulatory expectations.
Bioprocess Validation Market Opportunities
- Outsourcing Validation Services: There is an opportunity for service providers to offer validation services by outsourcing to specialized contract research organizations (CROs) and contract manufacturing organizations (CMOs), enhancing efficiency.
- Efficiency Through Digital Technologies: The use of digital technologies like AI and machine learning will improve work processes, enabling efficient data analysis and sound decision-making in bioprocess validation.
- Growing Demand for Advanced Solutions: As the biopharmaceutical industry expands, the demand for advanced, efficient, and robust bioprocess validation solutions will continue to rise, opening new markets for industrial players.
Bioprocess Validation Market Regional Insights
The regional trends and factors influencing the Bioprocess Validation Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Bioprocess Validation Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Bioprocess Validation Market
Bioprocess Validation Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ XX million |
| Market Size by 2031 | US$ XX Million |
| Global CAGR (2025 - 2031) | 14.5% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Test Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
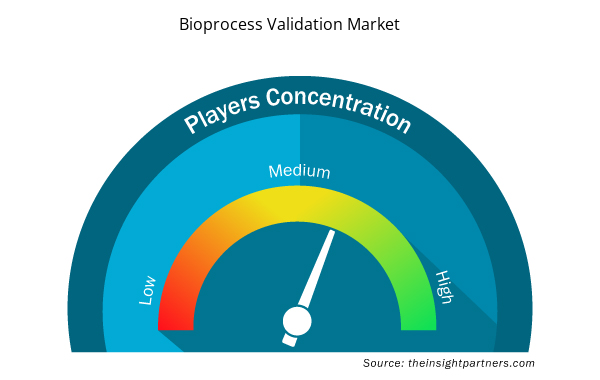
Bioprocess Validation Market Players Density: Understanding Its Impact on Business Dynamics
The Bioprocess Validation Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Bioprocess Validation Market are:
- Merck KGaA
- Pall Corporation (A Part of Danaher Corporation)
- Sartorius Stedim Biotech S.A.
- SGS S.A.
- Eurofins Scientific
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Bioprocess Validation Market top key players overview
Key Selling Points
- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the Bioprocess Validation Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the Bioprocess Validation Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
This text is related
to segments covered.

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
The leading players of the market are: Merck KGaA, Pall Corporation (A Part of Danaher Corporation), Sartorius Stedim Biotech S.A., SGS S.A., Eurofins Scientific, Cobetter Filtration Equipment Co., Ltd., Toxikon Corporation, Doc S.R.L., Meissner Filtration Products, Inc., Thermo Fisher Scientific, Inc.
The report can be delivered in PDF/PPT format; we can also share excel dataset based on the request.
Some of the customization options available based on request are additional 3-5 company profiles and country-specific analysis of 3-5 countries of your choice. Customizations are to be requested/discussed before making final order confirmation, as our team would review the same and check the feasibility.
Validation process will change dramatically with the advancements of artificial intelligence and machine learning of digital technologies, allowing users to get the predictive analytics, real-time monitoring, and automatic analysis of data. This trend of personalized medicine, along with cell and gene therapies, calls for more sophisticated and customized validation strategies. Sustainable issues and awareness about increasing environmental impacts will demand greener bioprocesses, which will have to be thoroughly validated to ensure proper regulatory compliance. This would ensure sustained growth in the market because demand for robust bioprocess validation solutions will stay strong as the industry continues to grow with the biopharmaceuticals industry.
Bioprocess Validation Market is expected to grow at a CAGR of 14.5% between 2023-2031
Major factors driving the Bioprocess Validation market include the increasing demand for biologics and biosimilars. These products by nature are regulated by stricter regulatory compliance and rigorous validation. Furthermore, advances in biomanufacturing, such as single-use systems and continuous manufacturing, need to be validated to ensure quality and consistency of final products. Additionally, with the increase in personal medicine and cell and gene therapies-based products requiring complex manufacturing, there is a better scope for strong bioprocess validation. The Bioprocess Validation market is likely to experience lucrative growth and change in the following years.
Trends and growth analysis reports related to Life Sciences : READ MORE..
1. Merck KGaA
2. Pall Corporation (A Part of Danaher Corporation)
3. Sartorius Stedim Biotech S.A.
4. SGS S.A.
5. Eurofins Scientific
6. Cobetter Filtration Equipment Co., Ltd.
7. Toxikon Corporation
8. Doc S.R.L.
9. Meissner Filtration Products, Inc.
10. Thermo Fisher Scientific, Inc.
 Get Free Sample For
Get Free Sample For