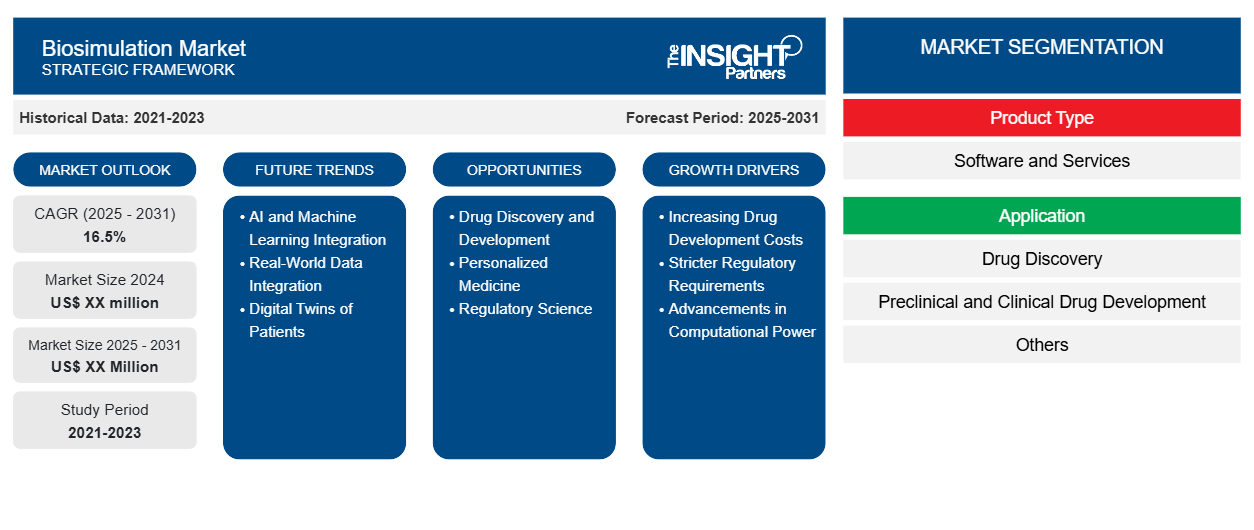
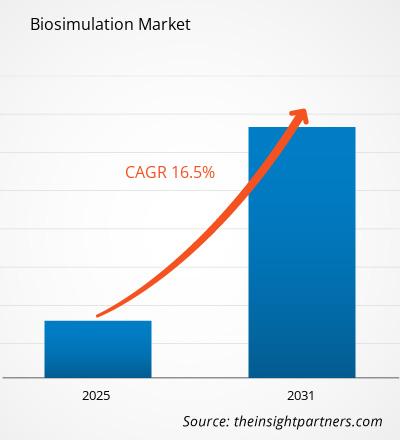
The Biosimulation Market is expected to register a CAGR of 16.5% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The report is segmented based on Product Type, Application, End User, and Geography. The global analysis is further broken down at the regional level and major countries. The market evaluation is presented in US$ for the above segmental analysis.
Purpose of the Report
The report Biosimulation Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
Biosimulation Market Segmentation
Product Type
- Software and Services
Application
- Drug Discovery
- Preclinical and Clinical Drug Development
- Others
End Users
- Pharmaceutical and Biotechnology Companies
- Research Institutes
- Contract Research Organizations
- Others
Geography
- North America
- Europe
- Asia-Pacific
- South and Central America
- Middle East and Africa
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Biosimulation Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Biosimulation Market Growth Drivers
- Increasing Drug Development Costs: Biosimulation can reduce costs by optimizing drug development processes.
- Stricter Regulatory Requirements: Rigorous regulatory standards necessitate robust preclinical data, which biosimulation can provide.
- Advancements in Computational Power: Increased computational power enables more complex and accurate simulations.
Biosimulation Market Future Trends
- AI and Machine Learning Integration: AI and ML will enhance the predictive capabilities of biosimulation models.
- Real-World Data Integration: Incorporating real-world data into simulations will improve their accuracy and relevance.
- Digital Twins of Patients: Creating virtual representations of patients to personalize treatment strategies.
Biosimulation Market Opportunities
- Drug Discovery and Development: Accelerating drug discovery and development by predicting drug efficacy and toxicity.
- Personalized Medicine: Tailoring treatments to individual patients based on their unique biological characteristics.
- Regulatory Science: Supporting regulatory decision-making by providing robust scientific evidence.
Biosimulation Market Regional Insights
The regional trends and factors influencing the Biosimulation Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Biosimulation Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Biosimulation Market
Biosimulation Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ XX million |
| Market Size by 2031 | US$ XX Million |
| Global CAGR (2025 - 2031) | 16.5% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Product Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Biosimulation Market Players Density: Understanding Its Impact on Business Dynamics
The Biosimulation Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Biosimulation Market are:
- Simulations Plus
- Certara USA, Inc.
- Schrödinger, LLC
- Dassault Systèmes
- Rosa & Co., LLC
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Biosimulation Market top key players overview
Key Selling Points
- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the Biosimulation Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the Biosimulation Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Product Type, Application, End Users, and Geography

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
Some of the customization options available based on request are additional 3-5 company profiles and country-specific analysis of 3-5 countries of your choice. Customizations are to be requested/discussed before making final order confirmation, as our team would review the same and check the feasibility.
The report can be delivered in PDF/PPT format; we can also share excel dataset based on the request.
The leading players in the Biosimulation Market are: Simulations Plus, Certara USA Inc, Schr dinger LLC, Dassault Syst mes, Rosa Co LLC, Genedata AG, Leadscope Inc, Evidera, Advanced Chemistry Development
Biosimulation Market is expected to grow at a CAGR of 16.5% between 2023-2031
The future trends of the Biosimulation Market are: Integration of AI and Machine Learning, Personalized Medicine
The driving factors impacting the Biosimulation Market are: Rising Drug Development Costs, Advancements in Computational Power and Modeling Techniques
Trends and growth analysis reports related to Technology, Media and Telecommunications : READ MORE..
The List of Companies
1. Simulations Plus
2. Certara USA, Inc.
3. Schrödinger, LLC
4. Dassault Systèmes
5. Rosa & Co., LLC
6. Genedata AG
7. Leadscope, Inc.
8. Evidera
9. Advanced Chemistry Development
10. Insilico Biotechnology
11. Chemical Computing Group
12. Certara
13. Physiomics
14. Nuventra Pharma
15. Inosim Software
 Get Free Sample For
Get Free Sample For