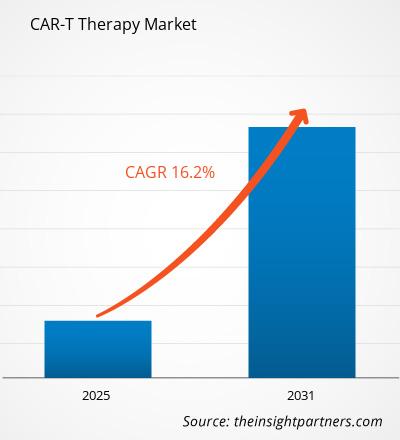
The CAR-T Therapy Market is expected to register a CAGR of 16.2% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The report is segmented by Targeted Antigen (CD 19,BCMA,HER2,GD2,CD 20,CD22,CD30,CD33,HER1 and Others). Further, the market is segmented on the basis of Therapeutic Application (Acute Lymphocytic Leukemia, Chronic Lymphocytic Leukemia, Diffuse Large B-Cell Lymphoma, Follicular Lymphoma, Multiple Myeloma, Glioblastoma, Sarcoma, Neuroblastoma, Acute Myeloid Leukemia, Breast Cancer, Pancreatic Cancer, Hepatocellular Carcinoma, Colorectal Cancer and Others). The global analysis is further broken-down at regional level and major countries. The Report Offers the Value in USD for the above analysis and segments.
Purpose of the Report
The report CAR-T Therapy Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
CAR-T Therapy Market Segmentation
Targeted Antigen
- CD 19
- BCMA
- HER2
- GD2
- CD 20
- CD22
- CD30
- CD33
- HER1 and Others
Therapeutic Application
- Acute Lymphocytic Leukemia
- Chronic Lymphocytic Leukemia
- Diffuse Large B-Cell Lymphoma
- Follicular Lymphoma
- Multiple Myeloma
- Glioblastoma
- Sarcoma
- Neuroblastoma
- Acute Myeloid Leukemia
- Breast Cancer
- Pancreatic Cancer
- Hepatocellular Carcinoma
- Colorectal Cancer and Others
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
CAR-T Therapy Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
CAR-T Therapy Market Growth Drivers
- Incidence of Cancer: According to World Health Organization (WHO), cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020. According to the International Agency of Research on Cancer, in 2040, 30.3 million new cases will be added to the global cancer burden. Chimeric antigen receptor (CAR) T-cell therapies can potentially treat new type of cancer treatment that uses the immune system to fight cancer. CAR T involves genetically engineering T cells to express a chimeric antigen receptor targeting a specific tumor antigen. Thus, the growing burden of cancer is driving the CAR-T therapy market.
- CAR-T approvals: Globally, cell therapies are widely adopted owing to the availability FDA approved products. In February 2022, the US Food and Drug Administration (FDA) approved Yescarta (axicabtagene ciloleucel) CAR T-cell therapy for adult patients with large B-cell lymphoma that is refractory to first line chemoimmunotherapy or that relapses within 1 month of first line chemoimmunotherapy. Yescarta is the first CAR T-cell therapy to receive a National Comprehensive Cancer Network (NCCN) Category 1 recommendation.
- CAR-T cell manufacturing: Latest developments in CAR-T cell manufacturing are efficiency-driven, scalable, and cost-effective. Reduced human error to improve throughput is a result of automation at the stage of cell processing, therefore, having faster production cycles. Modular facilities have the ability to be flexible and scaled rapidly as needed to meet demand without large capital investments.
CAR-T Therapy Market Future Trends
- Rising Number of CAR T-Cell Therapies in Clinical Trials: The current landscape for CAR T-cell therapies is promising, with multiple drug approvals, a rich pipeline, and many ongoing clinical trials. Pipelines of CAR T-cell therapies are in various stages of clinical development; major pharmaceutical companies are working to advance the pipeline space and future growth potential of the CAR T-cell therapy competitive domain. JW Therapeutics drug JWCAR029 is a CAR T cell product that targets CD19 and is intended to treat advanced lymphoma and leukemia. The molecule is currently in Phase II of development. JWCAR029 is initially being investigated for treating B-cell malignancies, focusing on relapsed and refractory DLBCL.
- Focus on Safety: Accordingly, ensuring confidence in such novel treatments also requires that improvements be made to the outcomes from CAR-T therapy by focusing on safety. Research is thus conducted on predictive measures, prevention, and management of one of the common side effects associated with CAR-T treatment, CRS. This includes improvement in protocols concerning the selection and monitoring of patients.
- Biomarker Development: Biomarkers for CAR-T therapy research should be regarded as an important factor that tends to improve the aspects of patient selection and treatment outcomes. This approach aids in knowing the effectiveness of CAR-T therapies and bringing in provisions for the adjustment in a timely manner as deemed necessary. The unification of academia, industry, and clinical research is important for helping in advancing the discovery and validation of new biomarkers that bring innovation into the field of CAR-T therapy.
CAR-T Therapy Market Opportunities
- Growing Investment in CAR-T Therapy: Companies operating in the T: cell therapy market focus on strategic developments such as collaborations, expansions, agreements, and investment, which help them improve their sales, expand their geographic reach, and enhance their capacities to cater to a larger than existing customer base. In August 2023, Astellas invested US$ 50 million in Poseida’s CAR T-cell therapy. Astellas will have exclusive negotiation and first refusal for licensing P: MUC1C: ALLO
- in solid tumors as part of the agreement. P: MUC1C: ALLO
- is an allogeneic CAR T- cell therapy targeting cancer: specific Mucin
- protein (MUC1: C) forms. It is currently under investigation in an open: label multicentre Phase I clinical trial (NCT05239143) treating multiple epithelial: derived solid tumor indications.
- Emerging Markets: Rising instances of oncology and increasing health expenses further point to huge potential in emerging markets for CAR-T therapy. The Asia-Pacific region and Latin America are also witnessing a high incidence of cancer cases, thus generating demand for advanced treatments like CAR-T. Local collaboration with international pharmaceutical giants helps in adapting CAR-T technologies to the needs of the local markets and conditions. Affordable CAR-T therapies tailor: made for emerging markets can greatly enhance access and adoption among patients.
- Expansion into Solid Tumors: Various approaches are under investigation to overcome this immunosuppressive tumor microenvironment, including the combination of CAR-T with checkpoint inhibitors. Several CAR-T therapies for solid tumors are being evaluated in various clinical trials that are ongoing to determine their efficacy and safety. These will not only identify potential modifications needed to achieve efficacy but also help determine how best to individualize CAR-T therapies by tailoring them to an individual patient's specific tumor profile, improving response rates, and minimizing adverse effects. Such combinations represent huge opportunities for the expansion of CAR-T therapy to include solid tumors.
CAR-T Therapy Market Regional Insights
The regional trends and factors influencing the CAR-T Therapy Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses CAR-T Therapy Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for CAR-T Therapy Market
CAR-T Therapy Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ XX million |
| Market Size by 2031 | US$ XX Million |
| Global CAGR (2025 - 2031) | 16.2% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Targeted Antigen
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
CAR-T Therapy Market Players Density: Understanding Its Impact on Business Dynamics
The CAR-T Therapy Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the CAR-T Therapy Market are:
- Novartis International AG
- Kite Pharma, Inc. (Gilead Sciences, Inc.)
- Juno Therapeutics (Celgene Corporation)
- Bluebird Bio, Inc. (Celgene Corporation)
- Sorrento Therapeutics Inc.
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the CAR-T Therapy Market top key players overview
Key Selling Points
- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the CAR-T Therapy Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the CAR-T Therapy Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
This text is related
to segments covered.

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
Players operating in the market are Bristol Myers Squibb, Immunocore Holdings, Legend Biotech, Jenssen Global Services, Gilead Sciences, Bluebird Bio, Novatis AG, and JW Therapeutics
North America region dominated the CAR-T therapy market in 2023.
Growing Investment in CAR-T Therapy act as a opportunity for growth of the market in forecast period.
The CAR-T Therapy Market is estimated to witness a CAGR of 16.2% from 202#to 2031
The major factors driving the CAR-T therapy market are:
1. Incidence of Cancer
2.CAR-T approvals
Acute Lymphocytic Leukemia segment, by therapeutic appication, dominated the market in 2023.
Trends and growth analysis reports related to Life Sciences : READ MORE..
1. Novartis International AG
2. Kite Pharma, Inc. (Gilead Sciences, Inc.)
3. Juno Therapeutics (Celgene Corporation)
4. Bluebird Bio, Inc. (Celgene Corporation)
5. Sorrento Therapeutics Inc.
6. Mustang Bio, Inc
7. Aurora Biopharma Inc.
8. Legend Biotech (Genscript Biotech Corporation)
9. Pfizer, Inc.
10. CARsgen Therapeutics, Ltd.
 Get Free Sample For
Get Free Sample For