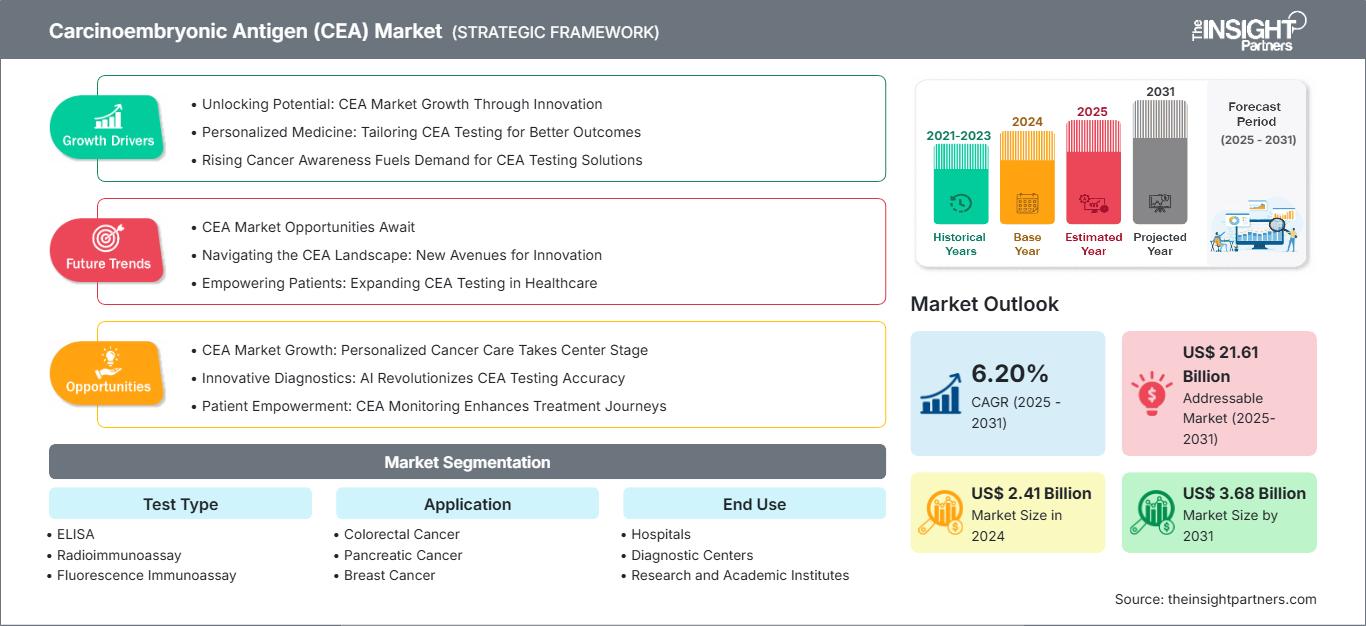
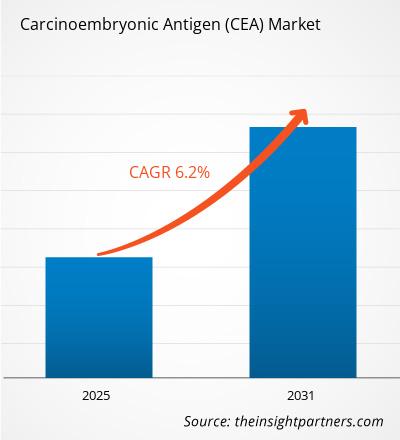
The Carcinoembryonic Antigen (CEA) Market is expected to register a CAGR of 6.20% from 2025 to 2031, with a market size expanding from US$ 2.41 Billion in 2024 to US$ 3.68 Billion by 2031.
The report is categorized by Test Type (ELISA, Radioimmunoassay, Fluorescence Immunoassay) and further analyzes the market based on Application (Colorectal Cancer, Pancreatic Cancer, Breast Cancer, Lung Cancer). It also examines the market by End Use (Hospitals, Diagnostic Centers, Research and Academic Institutes) . A comprehensive breakdown is provided at global, regional, and country levels for each of these key segments.
The report includes market size and forecasts across all segments, presenting values in USD. It also delivers key statistics on the current market status of leading players, along with insights into prevailing market trends and emerging opportunities.
Purpose of the Report
The report Carcinoembryonic Antigen (CEA) Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
Carcinoembryonic Antigen (CEA) Market Segmentation
Test Type
- ELISA
- Radioimmunoassay
- Fluorescence Immunoassay
Application
- Colorectal Cancer
- Pancreatic Cancer
- Breast Cancer
- Lung Cancer
End Use
- Hospitals
- Diagnostic Centers
- Research and Academic Institutes
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Carcinoembryonic Antigen (CEA) Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Carcinoembryonic Antigen (CEA) Market Growth Drivers
- Unlocking Potential: CEA Market Growth Through Innovation
- Personalized Medicine: Tailoring CEA Testing for Better Outcomes
- Rising Cancer Awareness Fuels Demand for CEA Testing Solutions
Carcinoembryonic Antigen (CEA) Market Future Trends
- CEA Market Opportunities Await
- Navigating the CEA Landscape: New Avenues for Innovation
- Empowering Patients: Expanding CEA Testing in Healthcare
Carcinoembryonic Antigen (CEA) Market Opportunities
- CEA Market Growth: Personalized Cancer Care Takes Center Stage
- Innovative Diagnostics: AI Revolutionizes CEA Testing Accuracy
- Patient Empowerment: CEA Monitoring Enhances Treatment Journeys
Carcinoembryonic Antigen (CEA) Market Regional Insights
The regional trends and factors influencing the Carcinoembryonic Antigen (CEA) Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Carcinoembryonic Antigen (CEA) Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Carcinoembryonic Antigen (CEA) Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ 2.41 Billion |
| Market Size by 2031 | US$ 3.68 Billion |
| Global CAGR (2025 - 2031) | 6.20% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Test Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Carcinoembryonic Antigen (CEA) Market Players Density: Understanding Its Impact on Business Dynamics
The Carcinoembryonic Antigen (CEA) Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Carcinoembryonic Antigen (CEA) Market top key players overview
Key Selling Points
- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the Carcinoembryonic Antigen (CEA) Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the Carcinoembryonic Antigen (CEA) Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends





















 Get Free Sample For
Get Free Sample For