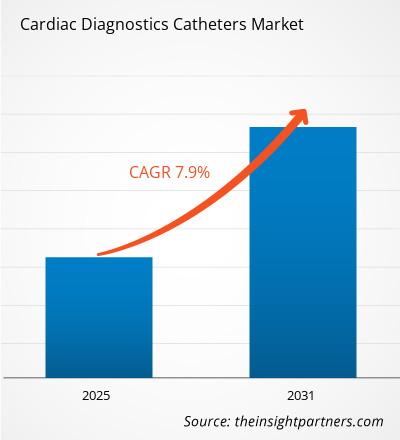
The Cardiac Diagnostics Catheters Market is expected to register a CAGR of 7.90% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The report is segmented by Cardiac Diagnostics Catheters Market By Product (Diagnostic imaging Catheters, Non-imaging Diagnostic Catheters); Indications (Coronary artery disease, Evaluate the left ventricular function, Assessment of pericardial and myocardial diseases, Assessment of congenital heart diseases, Evaluation of heart failure); End-User (Hospitals, Diagnostic Centers, Others) , and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America). The global analysis is further broken-down at regional level and major countries. The Report Offers the Value in USD for the above analysis and segments.
Purpose of the Report
The report Cardiac Diagnostics Catheters Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
Cardiac Diagnostics Catheters Market Segmentation
Product
- Diagnostic imaging Catheters
- Non-imaging Diagnostic Catheters
Indications
- Coronary artery disease
- Evaluate the left ventricular function
- Assessment of pericardial and myocardial diseases
- Assessment of congenital heart diseases
- Evaluation of heart failure
End-User
- Hospitals
- Diagnostic Centers
- Others
Geography
- North America
- Europe
- Asia-Pacific
- South and Central America
- Middle East and Africa
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Cardiac Diagnostics Catheters Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Cardiac Diagnostics Catheters Market Growth Drivers
- Increasing Prevalence of Cardiovascular Diseases:The market for cardiac diagnostic catheters is primarily driven by the increase in cardiovascular diseases (CVDs). The World Health Organization states that cardiovascular diseases (CVDs) are the world's largest cause of mortality, and that in order to successfully manage these disorders, improved diagnostic techniques are required. For instance, cardiovascular disease claims one life in the United States every 34 seconds, underscoring the critical need for diagnostic procedures.
- Aging Population: Because older people are more prone to heart-related disorders, the world aging population is contributing to an increased incidence of cardiovascular diseases. As healthcare systems adjust to the needs of elderly people, this demographic shift is anticipated to increase demand for cardiac diagnostic catheters.
- Rise in Healthcare Spending:Globally, rising healthcare costs are making it easier to obtain cutting-edge medical equipment, such as heart diagnostic catheters. The availability and use of these gadgets are anticipated to increase as healthcare organizations make greater investments in creative solutions. Such a factor has assisted the overall market growth in the recent past and is expected to continue a similar trend during the forecast period.
Cardiac Diagnostics Catheters Market Future Trends
- Technological Developments:Catheter technological advancements are greatly improving diagnostic capacities. In line with patients' and healthcare professionals' increasing preference for minimally invasive procedures, newer models are made to be more effective and less intrusive. Furthermore, developments like ultrasound-guided and MRI-compatible catheters are increasing the precision and accuracy of diagnosis.
- Growing Awareness of Early Detection:Patients and healthcare professionals alike are becoming more conscious of the significance of early cardiovascular disease detection and treatment. The need for efficient diagnostic instruments, such as catheters that can deliver prompt and precise evaluations, is being driven by this awareness.Such a factor has assisted the overall market growth in the recent past and is expected to continue a similar trend during the forecast period.
- Healthcare Infrastructure Improvements:In many areas, particularly North America, investments in healthcare infrastructure and the use of cutting-edge medical technologies are common. Sophisticated cardiac catheter products are being integrated by hospitals more frequently in an effort to raise the quality of patient care and service offerings.Such a factor has assisted the overall market growth in the recent past and is expected to continue a similar trend during the forecast period.
Cardiac Diagnostics Catheters Market Opportunities
- Integration of Advanced Imaging Technologies: Internal imaging has become more accurate and detailed since diagnostic catheters have been equipped with imaging modalities like CT, MRI, and ultrasound. Better visualization during procedures is made possible by this, which results in more accurate diagnosis and treatments. For example, the high-resolution imaging capabilities of optical coherence tomography (OCT) have made it popular, especially in cardiology.
- Shift Towards Minimally Invasive Techniques: Catheters that enable less invasive operations have been made possible by technological advancements. These methods improve patient comfort, shorten recovery periods, and decrease the likelihood of problems. Because of this, patients and healthcare professionals are increasingly choosing catheter-based diagnostics over conventional surgical techniques.
- Development of Smart Catheters:Patient care is changing as a result of the introduction of smart catheters with sensors for real-time monitoring. During procedures, these devices may measure a variety of physiological indicators, enabling quick adjustments and better results. For instance, they can improve procedure safety and effectiveness by offering vital information on blood flow and pressure during cardiac operations.
Cardiac Diagnostics Catheters Market Regional Insights
The regional trends and factors influencing the Cardiac Diagnostics Catheters Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Cardiac Diagnostics Catheters Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Cardiac Diagnostics Catheters Market
Cardiac Diagnostics Catheters Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ XX million |
| Market Size by 2031 | US$ XX Million |
| Global CAGR (2025 - 2031) | 7.90% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Product
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Cardiac Diagnostics Catheters Market Players Density: Understanding Its Impact on Business Dynamics
The Cardiac Diagnostics Catheters Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Cardiac Diagnostics Catheters Market are:
- Abbott Laboratories
- Boston Scientific Corporation
- Ares Medikal
- Medtronic plc
- BrosMed Medical Co. Ltd.
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Cardiac Diagnostics Catheters Market top key players overview
Key Selling Points
- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the Cardiac Diagnostics Catheters Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the Cardiac Diagnostics Catheters Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset


- Medical and Research Grade Collagen Market
- Resistance Bands Market
- Occupational Health Market
- Electronic Toll Collection System Market
- Asset Integrity Management Market
- Educational Furniture Market
- Medical Second Opinion Market
- Lyophilization Services for Biopharmaceuticals Market
- Aerosol Paints Market
- Hair Extensions Market

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
This text is related
to segments covered.

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
In Europe, the UK held the second largest share in 2023.
Asia pacific region is expected to witness the highest growth during the forecast period
Integration of Advanced Imaging Technologies
The Cardiac Diagnostics Catheters Market is estimated to witness a CAGR of 7.90% from 2023 to 2031
Increasing Prevalence of Cardiovascular Diseases
North America held the largest share in 2023 and is expected to retain its dominance during the forecast period
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies
1. Abbott Laboratories
2. Boston Scientific Corporation
3. Ares Medikal
4. Medtronic plc
5. BrosMed Medical Co. Ltd.
6. Cascade Health Care
7. Johnson & Johnson
8. Koninklijke Philips N.V.
9. Merit Medical Systems
10. Teleflex Incorporated
 Get Free Sample For
Get Free Sample For