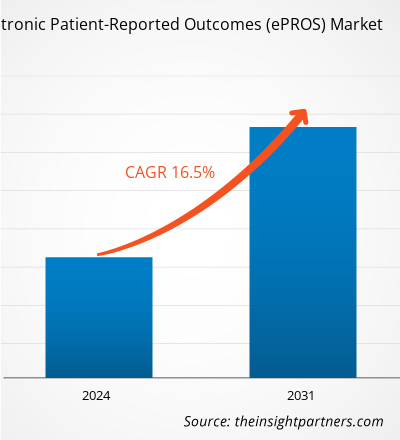
The electronic patient-reported outcomes (ePROS) market size is projected to reach US$ 2,868.00 million by 2031 from US$ 843.77 million in 2023. The market is expected to register a CAGR of 16.5% during 2023–2031. The integration of artificial intelligence and machine learning with ePROS platforms is likely to act as a future trend in the market.
Electronic Patient-Reported Outcomes (ePROS) Market Analysis
The electronic patient-reported outcomes (ePROS) market is mainly driven by continuous efforts made by pharmaceutical and biotechnology sectors and medical device companies for product innovations. Other factors contributing to the market expansion include the globalization of clinical trials, rapid advancements in associated technologies, and an increase in demand for CROs for conducting clinical trials. Furthermore, conferences such as Global Clinical Trials Connect 2022 offer a platform to companies in this marketplace. The platform helps companies get acquainted with advancements in clinical trials and clinical research.
Electronic Patient-Reported Outcomes (ePROS) Market Overview
Market players are taking various strategic initiatives such as product launches, product approvals, and collaborations, which are further expected to support the market growth. For instance, in June 2024, Evidation launched MigraineSmart, which is a health engagement experience and symptom tracking program on the Evidation app that harnesses survey data, electronic patient-reported outcomes (ePROs), wearable data, and evidence-based content to make it possible for individuals to better understand and manage their migraines. Therefore, an increase in digital health technologies, a surge in demand for patient-centered care, and a rise in strategic initiatives by market players are expected to fuel the growth of the electronic patient-reported outcomes (ePROs) market in the coming years.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Electronic Patient-Reported Outcomes (ePROS) Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Electronic Patient-Reported Outcomes (ePROS) Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Electronic Patient-Reported Outcomes (ePROS) Market Drivers and Opportunities
Emerging Applications of Electronic Patient-Reported Outcomes (ePROS)
The emerging applications of electronic patient-reported outcomes (ePRO) drive the market. The new uses of ePRO systems in decentralized clinical trials, remote patient monitoring, and other areas are stimulating innovation and extending the use of patient-reported outcomes in various healthcare, research, and clinical settings. The adoption of ePRO systems is driven by decentralized clinical trials (DCTs) growth. Patients can participate in DCTs remotely from the comfort of their homes, and ePRO tools are widely used for collecting patient-reported data immediately. The transition to virtual trials improves patient recruitment and retention; lowers the need for in-person visits; and increases trial efficiency of various diseases, including rare diseases, across geographically dispersed populations. In addition, the players are making strategic developments to meet the customers' needs. For instance, in June 2023, uMotif, a clinical trial technology company, and Syneos Health, an integrated biopharmaceutical solution company, announced a strategic partnership. By developing a more effective, end-to-end digital platform with electronic solid clinical outcome assessment (eCOA) and electronic patient-reported outcomes (ePRO) capabilities—this partnership helped accelerate clinical trials and expedite the delivery of new therapies to patients.
ePRO systems are being used to monitor acute conditions such as post-operative recovery and chronic conditions, including diabetes and hypertension, as healthcare increasingly moves toward telemedicine and remote patient monitoring (RPM). To help healthcare providers make regular interventions and changes to care plans, patients can report symptoms, medication adherence, and treatment responses from the comfort of their own homes. This approach lowers the rate of hospital readmissions, enhances chronic illness management, and improves patient satisfaction by offering ongoing, individualized care without requiring frequent in-person visits.
The adoption of ePRO systems is propelled by the growing use of wearables, including smartwatches, fitness trackers, and Internet of Things (IoT) devices in the healthcare industry. Predictive healthcare interventions can be made possible by combining objective data from wearables with subjective patient-reported outcomes. This results in better condition monitoring and proactive condition management. Pharmaceutical companies are utilizing ePRO systems to monitor adverse events in real-world settings, helping them gain traction in pharmacovigilance and post-market surveillance, thereby ensuring long-term drug safety and efficacy while adhering to regulatory requirements.
Rising Adoption of Telehealth and Remote Monitoring to Offer Opportunity for Market Growth
The market for electronically reported patient outcomes (ePROs) is expected to experience lucrative opportunities with the rising adoption of telehealth and remote monitoring for clinical trials during the forecast period. Patients in remote or underserved areas can report real-time information from the comfort of their homes owing to telehealth and remote monitoring solutions, which increase the accessibility of patient data. Health data can be continuously transmitted via remote monitoring devices, such as wearables and linked health systems. When coupled with ePRO systems, medical professionals receive detailed information regarding a patient's condition in real time, enabling more precise and regular interventions. For example, Vivalink's integrated acute remote patient monitoring solution provides continuous and real-time monitoring of patient vitals, including live ECG, as well as information about vitals and biometrics, EPRO/ECOA/surveys, centralized data services, remote data collection, and patient adherence. The efficacy of ePRO systems is expanded by telehealth and remote monitoring developments, establishing them as a crucial element of patient-centered care.
Electronic Patient-Reported Outcomes (ePROS) Market Report Segmentation Analysis
Key segments that contributed to the derivation of the electronic patient-reported outcomes (ePROS) market analysis are delivery mode, application, and end user.
- The electronic patient-reported outcomes (ePROS) market, based on delivery mode, is divided into cloud based and on-premise. The cloud based segment held a largest share of the market in 2023.
- By application, the market is segmented into oncology, respiratory, and others. The oncology segment held the largest share of the market in 2023.
- Based on end user, the electronic patient-reported outcomes (ePROS) market is segmented into CROs, pharmaceutical companies, and others. The pharmaceutical companies segment dominated the market in 2023.
Electronic Patient-Reported Outcomes (ePROS) Market Share Analysis by Geography
The geographic scope of the electronic patient-reported outcomes (ePROS) market report is mainly divided into five regions: North America, Asia Pacific, Europe, South & Central America, and the Middle East & Africa. In terms of revenue, North America dominated the market in 2023. The market in this region is mainly driven by the presence of developed healthcare infrastructure, increased focus on digital health, and rise in product launches and other strategic initiatives by market players. The US is the largest and fastest-growing market for electronic patient-reported outcomes (ePROS). The country accounts for the major market share owing to the increasing use of digital health technologies, which helps provide patient-centered care. This involves integrating healthcare services with software and hardware, which can be managed and monitored with the help of apps, wearable devices, and other devices. The increasing focus on chronic disease management has increased the demand for ePROs as they allow continuous assessment and timely interventions. As per the article published by the American Society of Clinical Oncology in June 2023, in the US, ePRO inclusion in the Centers for Medicare and Medicaid Innovation's alternative payment model pilot, the Enhancing Oncology Model, is a sign that ePRO implementation should be a crucial step toward high-quality and transformational cancer care that is likely to foster broader adoption. The surging number of clinical trials in the country is expected to increase the demand for ePROs during the forecast period.
Electronic Patient-Reported Outcomes (ePROS) Market Regional Insights
The regional trends and factors influencing the Electronic Patient-Reported Outcomes (ePROS) Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Electronic Patient-Reported Outcomes (ePROS) Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
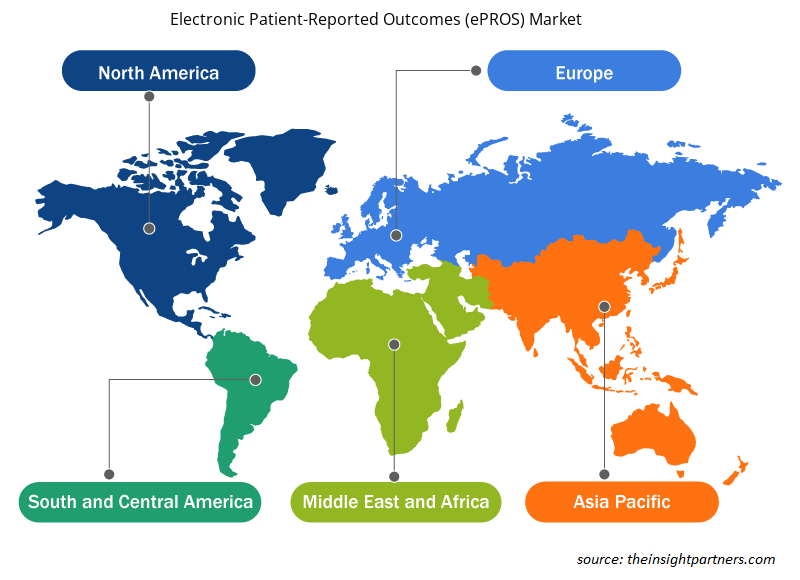
- Get the Regional Specific Data for Electronic Patient-Reported Outcomes (ePROS) Market
Electronic Patient-Reported Outcomes (ePROS) Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2023 | US$ 843.77 Million |
| Market Size by 2031 | US$ 2,868.00 Million |
| Global CAGR (2023 - 2031) | 16.5% |
| Historical Data | 2021-2022 |
| Forecast period | 2024-2031 |
| Segments Covered |
By Delivery Mode
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Electronic Patient-Reported Outcomes (ePROS) Market Players Density: Understanding Its Impact on Business Dynamics
The Electronic Patient-Reported Outcomes (ePROS) Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Electronic Patient-Reported Outcomes (ePROS) Market are:
- OpenClinica LLC
- Buddy Healthcare Ltd Oy
- Castor
- Medable Inc
- Assistek
- Signant Health
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Electronic Patient-Reported Outcomes (ePROS) Market top key players overview
Electronic Patient-Reported Outcomes (ePROS) Market News and Recent Developments
The electronic patient-reported outcomes (ePROS) market is evaluated by gathering qualitative and quantitative data post primary and secondary research, which includes important corporate publications, association data, and databases. A few of the developments in the market are listed below:
- Clinical Ink launched EDCXtra—an innovative Electronic Data Capture (EDC) system. EDCXtra incorporates all existing DDC functionality into a single web-based application, which includes Clinical ink’s industry-leading electronic Clinical Outcome Assessments (eCOAs) and eConsent solutions. This application provides a comprehensive, all-in-one GCP-compliant platform. (Source: Clinical Ink, Company Website, August 2024)
- PatientIQ launched ResearchPRO an electronic data capture (EDC) platform designed to streamline the process of launching and managing research studies. With the introduction of ResearchPRO, PatientIQ is able to provide a more dedicated and sophisticated solution set tailored specifically to the unique needs of clinical researchers, clinical research organizations (CROs), and industry sponsors. (Source: PatientIQ, Company Website, July 2024)
Electronic Patient-Reported Outcomes (ePROS) Market Report Coverage and Deliverables
The "Electronic Patient-Reported Outcomes (ePROS) Market Size and Forecast (2021–2031)" report provides a detailed analysis of the market covering below areas:
- Electronic patient-reported outcomes (ePROS) market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Electronic patient-reported outcomes (ePROS) market trends, as well as market dynamics such as drivers, restraints, and key opportunities
- Detailed PEST and SWOT analysis
- Electronic patient-reported outcomes (ePROS) market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments for the electronic patient-reported outcomes (ePROS) market
- Detailed company profiles
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset


- Playout Solutions Market
- Medical Second Opinion Market
- Pharmacovigilance and Drug Safety Software Market
- Fertilizer Additives Market
- Sandwich Panel Market
- Drain Cleaning Equipment Market
- Airline Ancillary Services Market
- Frozen Potato Market
- Nuclear Decommissioning Services Market
- Employment Screening Services Market

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
This text is related
to segments covered.

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
North America dominated the market in 2023.
OpenClinica LLC, Buddy Healthcare Ltd Oy, Castor, Medable Inc, Assistek, Signant Health, Crucial Data Solutions, Curebase, Y-Prime LLC, Clinical Ink Inc, Medidata Solutions, Veeva Systems Inc, Medrio, and PatientIQ are among the key players in the market.
The emerging applications of electronic patient-reported outcomes (ePROS) and increasing focus on patient-centered care activities are among the most significant factors fueling the market growth.
The electronic patient-reported outcomes (ePROS) market value is expected to reach US$ 2,868.00 million by 2031.
The market is expected to register a CAGR of 16.5% during 2023–2031.
Integration of artificial intelligence and machine learning with ePROS platforms is expected to be a prime trend in the market in the coming years.
Trends and growth analysis reports related to Technology, Media and Telecommunications : READ MORE..
The List of Companies - Electronic Patient-Reported Outcomes (ePROS) Market
- OpenClinica LLC
- Buddy Healthcare Ltd Oy
- Castor
- Medable Inc
- Assistek
- Signant Health
- Crucial Data Solutions
- Curebase
- Y-Prime LLC
- Clinical Ink Inc
 Get Free Sample For
Get Free Sample For