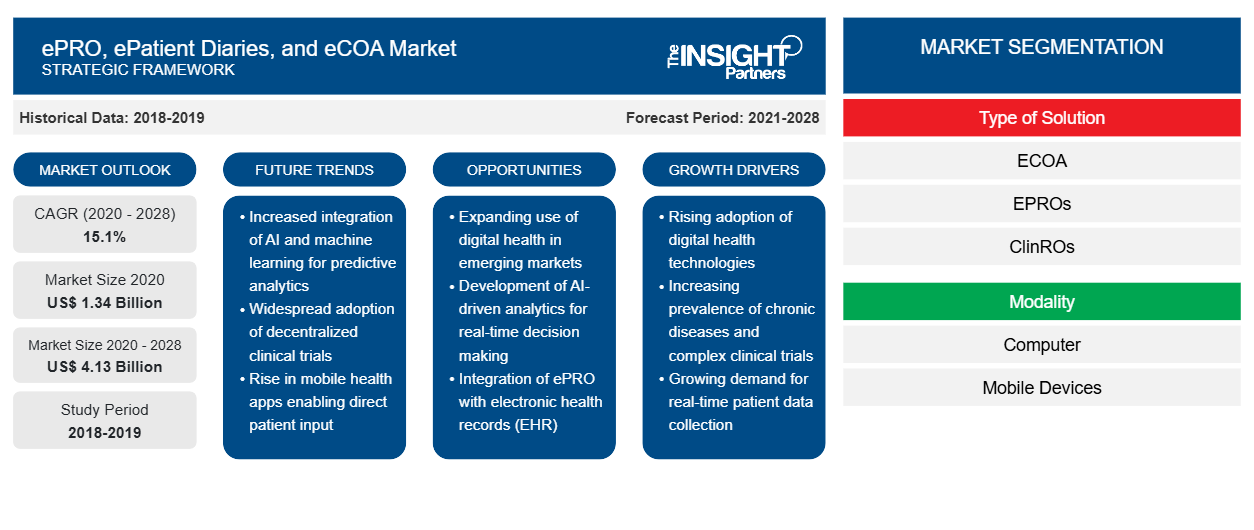
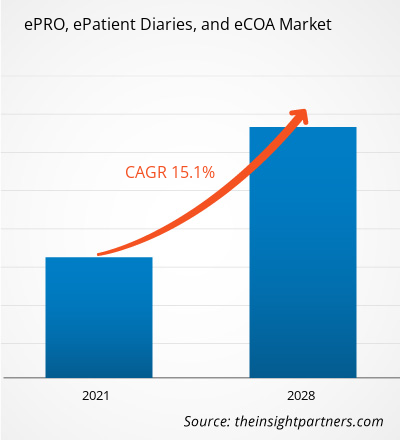
The ePRO, epatient diaries and eCOA market was valued at US$ 1,342.92 million in 2020, it is expected to grow at a CAGR of 15.1% during 2021-2028.
The usage of electronic Clinical Outcome Assessment (eCOA) is a push toward adapting the ‘new normal’ as it is a method to gather patient data electronically by using technologies such as smart home devices, handheld monitors, wearables, e-diaries, tablets, and web servers to allow the stakeholders (patients, healthy volunteers, investigators and caregivers) in the trials to report outcomes directly and digitally. Although historically COA was only related to the evaluation of Patient-Related Outcomes (PRO), the FDA has now broadened the definition to include PerfO, ClinRO, and ObsRO along with PRO. In simple terms, when the above parameters are reported electronically, they fit under the eCOA spectrum.
eCOA/ePRO platform has substantial benefits for sponsors and CROs, as it reduces administrative burden, mitigates cost, and fastens trials. Such system shows strong results with fewer errors and discrepancies, improved data quality, clearer signals, and standardized accurate studies. The factors such as the increasing adoption of EHR and government regulations mandating the maintenance of health records drive the growth of the ePRO, epatient diaries, and eCOA market.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
ePRO, ePatient Diaries, and eCOA Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
ePRO, ePatient Diaries, and eCOA Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Market Insights
Growing Demand for Clinical Trials
As per the data provided by Clinicaltrials.gov, 2016 has been a big one for clinical research. There were around 366,000 clinical studies registered globally as of February 2021. Clinical studies are an essential part of drug development worldwide. The number of clinical studies has augmented significantly since there were just 2,119 reported in 2000. Of these, almost half are drug or biological trials (123,806), with 45,501 currently recruiting participants. At present, there are 43 therapeutics in Phase II for COVID-19. Companies engaged in the development are AstraZeneca plc; Arch Biopartners Inc.; Applied Therapeutics Inc.; Apeiron Biologics GmbH; 4D Pharma plc; and AB Science SA. Companies are collaborating with other firms to hasten the development of therapeutics and vaccines. For instance, Eli Lilly partnered with AbCellera to develop vaccines; GSK, Novartis, and MSD are working with the Bill and Melinda Gates Foundation. GSK and Sanofi are working together to develop an adjuvanted COVID-19 vaccine.
Further, the outbreak of COVID-19 has created even greater urgency for clinical trials to become more virtual and deliver a safe, convenient patient experience. Thus, market players are launching new products, thereby driving the adoption of patient centric platforms. For instance, Veeva Systems announced MyVeeva for Patients, a new application for clinical research sites. With capabilities for virtual visits, patient adherence, ePRO, eConsent, eSource, and an easy-to-use ePRO, ePatient Diaries, and eCOA, MyVeeva for Patients will make it easier for clinical research sites to deliver a patient-centric and paperless clinical trial experience for patients and sponsors. Additionally, in January 2021 YPrime, LLC, announced the launch of their fifth-generation electronic clinical outcome assessment (eCOA) platform. Its newest platform features an improved user experience for patients, clinical investigators, sponsors, and CROs. This advanced technology enhances clinical trial efficiency, increases site satisfaction, and improves patient compliance. This release also ensures the delivery of cleaner data, streamlining the clinical trial data collection, and approval process.
Type of Solution-Based Insights
Based on type of solution, the ePRO, epatient diaries, and eCOA market is segmented into eCOA (Electronic Clinical Outcome Assessments), ePROs (Patient Reported Outcomes), ClinROs (Clinician Reported Outcomes), ObsROs (Observer Reported Outcomes), PerfOs (Performance Outcomes), E-Patient Diaries. The eCOA segment held the largest share of the market in 2020. However, the ePRO segment is estimated to register the highest CAGR in the market during the forecast period.
Modality Mode-Based Insights
Based on modality, the ePRO, epatient diaries, and eCOA market is bifurcated into computer and mobile devices. The mobile devices segment held a larger share of the market in 2020. Also, the same segment is estimated to register a higher CAGR in the market during the forecast period.
End User-Based Insights
Based on end user, the ePRO, epatient diaries, and eCOA market is segmented into clinical trial sponsors, contract research organizations (CROs), hospitals, academic institutes, pharmaceutical companies, and others. The clinical trial sponsors segment held the largest share of the market in 2020, and is estimated to register the highest CAGR of 15.5% in the market during the forecast period.
Various companies operating in the ePRO, epatient diaries, and eCOA market adopt strategies such as product launches, mergers and acquisitions, collaborations, product innovations, and product portfolio expansions to expand their footprint worldwide, maintain the brand name, and meet the growing demand from end users.
ePRO, e-Patient Diaries and eCOA ePRO, ePatient Diaries, and eCOA Market Regional Insights
The regional trends and factors influencing the ePRO, ePatient Diaries, and eCOA Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses ePRO, ePatient Diaries, and eCOA Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for ePRO, ePatient Diaries, and eCOA Market
ePRO, ePatient Diaries, and eCOA Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2020 | US$ 1.34 Billion |
| Market Size by 2028 | US$ 4.13 Billion |
| Global CAGR (2020 - 2028) | 15.1% |
| Historical Data | 2018-2019 |
| Forecast period | 2021-2028 |
| Segments Covered |
By Type of Solution
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
ePRO, ePatient Diaries, and eCOA Market Players Density: Understanding Its Impact on Business Dynamics
The ePRO, ePatient Diaries, and eCOA Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the ePRO, ePatient Diaries, and eCOA Market are:
- ERT Clinical
- ArisGlobal LLC
- The Diary Pte. Ltd
- ICON PLC
- PAREXEL INTERNATIONAL CORPORATION
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the ePRO, ePatient Diaries, and eCOA Market top key players overview
ePRO, ePatient diaries, and eCOA Market – by Type of Solution
- eClinical Outcome Assessments
- ePatient Reported Outcomes
- Clinician Reported Outcomes
- Observer Reported Outcomes
- Performance Outcomes
- ePatient Diaries
ePRO, ePatient diaries, and eCOA Market – by Modality
- Computer
- Mobile Devices
ePRO, ePatient diaries, and eCOA Market – by End User
- Clinical Trial Sponsors
- Contract Research Organizations
- Hospitals
- Academic Institutes
- Pharmaceutical Companies
- Others
ePRO, ePatient diaries, and eCOA Market – by Geography
- North America
- US
- Canada
- Mexico
- Europe
- France
- Germany
- Italy
- UK
- Spain
- Rest of Europe
- Asia Pacific (APAC)
- China
- India
- South Korea
- Japan
- Australia
- Rest of Asia Pacific
- Middle East & Africa (MEA)
- South Africa
- Saudi Arabia
- UAE
- Rest of Middle East & Africa
- South America (SAM)
- Brazil
- Argentina
- Rest of South and Central America
Company Profiles
- ERT Clinical
- ArisGlobal LLC
- The Diary Pte. Ltd
- ICON PLC
- PAREXEL INTERNATIONAL CORPORATION
- Anju Software, Inc.
- Kayentis
- Bracket Global LLC
- Dassault Systèmes SE
- CRF Health
- eClinical Solutions
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset


- Bioremediation Technology and Services Market
- Animal Genetics Market
- Hair Extensions Market
- Broth Market
- Sterilization Services Market
- Airline Ancillary Services Market
- Clinical Trial Supplies Market
- Social Employee Recognition System Market
- Enzymatic DNA Synthesis Market
- 3D Mapping and Modelling Market

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
ePRO ; Modality ); End User , and Geography

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, Saudi Arabia, South Africa, South Korea, Spain, United Arab Emirates, United Kingdom, United States
Trends and growth analysis reports related to Technology, Media and Telecommunications : READ MORE..
The List of Companies - ePRO ePatient Diaries and eCOA Market
- ERT Clinical
- ArisGlobal LLC
- The Diary Pte. Ltd
- ICON PLC
- PAREXEL INTERNATIONAL CORPORATION
- Anju Software, Inc.
- Kayentis
- Bracket Global LLC
- Dassault Systèmes SE
- CRF Health
- eClinical Solutions
 Get Free Sample For
Get Free Sample For