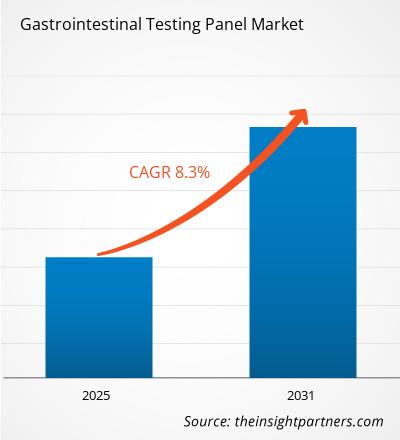
The Gastrointestinal Testing Panel Market is expected to register a CAGR of 8.3% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The report is segmented by Pathogen Type (Bacteria, Viruses, and Parasites). The report is segmented by Testing Type (Syndromic Full, Multiplex Panel, and Low Plex Panel). The report further presents analysis based on the End-use (Hospitals, Diagnostic Laboratories, and Others). The global analysis is further broken-down at regional level and major countries. The Report Offers the Value in USD for the above analysis and segments.
Purpose of the Report
The report Gastrointestinal Testing Panel Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
Gastrointestinal Testing Panel Market Segmentation
Pathogen Type
- Bacteria
- Viruses
- Parasites
Testing Type
- Syndromic Full
- Multiplex Panel
- Low Plex Panel
End-use
- Hospitals
- Diagnostic Laboratories
- Others
Geography
- North America
- Europe
- Asia-Pacific
- South and Central America
- Middle East and Africa
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Gastrointestinal Testing Panel Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Gastrointestinal Testing Panel Market Growth Drivers
- Gastrointestinal Disorders on the Rise: Like other categories of health and psychological health, the rise in the prevalence of gastrointestinal disorders is a key determinant of the expansion of this market. More and more regions of the globe are facing issues of irritable bowel syndrome, celiac disease, colorectal cancer, inflammatory bowel diseases (IBD) such as Crohn’s disease, and ulcerative colitis. There are changes in diets, more sedentary lifestyles, and increased levels of stress as the main factors that are increasing the incidences of gastrointestinal issues. A rise in the incidence of these diseases creates a corresponding increase in the need for precise reliable tests to assist in early diagnosis, monitoring, and treatment under various conditions.
- Improved Diagnostic Equipment: New Technologies - there is an ongoing transformation of GI testing due to the development of advanced molecular biology analyzers, genetic testing platforms, as well as non-invasive approaches. Currently available technologies include next generation sequencing NGS, PCR tests, and ELISA tests which have made it possible to test for different digestive tract diseases more effectively, more quickly, and less invasively. Improved technology for pathogen and biomarker detection has broadened the use of genetic alterations in GI testing panels thereby increasing the effectiveness of GI testing. The additional increase in point of care testing devices and home diagnostic kits availability is positively influencing the market development.
- Increased Focus on Preventive Healthcare: There has been a paradigm shift towards preventive healthcare, with individuals becoming more proactive in managing their health. The rising awareness about the importance of early detection of diseases like colorectal cancer and celiac disease has fueled demand for gastrointestinal testing panels. Such patients tend to use these tests even when they appear well due to the fears of severe gastrointestinal ailments. Healthcare providers
Gastrointestinal Testing Panel Market Future Trends
- Personalized and Precision Medicine: Diagnostic and therapeutic options are increasingly accepting a targeted, personalized and precision medicine in the management of the diseases of the gastrointestinal tract. This method customizes treatment to the individual patient based on genetics, environment and way of life. As such GI testing panels are being developed with some additional biomarkers that would enhance some accuracy in diagnosis and treatment levels. These tests can identify genetic factors, dysbiosis, and other characteristics most suited to the patient and help in choosing the right treatment especially in cases of IBS, Crohn’s disease, and cancers involving the colon.
- Integration of Artificial Intelligence (AI) and Machine Learning: AI and ' cloud based on AI' machine learning find more application in GI diagnostics with a view to improving the accuracy of the test results and the decisions to be made clinically. The advanced technology allows for the use of AI algorithms to sift through years of GI testing panels data, make models and forecast diseases quicker and better than the manual approaches. It is observable say in the analysis of endoscopic images whereby AI is used to look for and help mark areas with abnormal tissue or any tumors, thus aiding the physician’s judgment on the best course of treatment to take.
- Point-of-Care Testing and Home-Based Diagnostics: There is a rising tendency for point of care (POC) diagnostics and at home testing. The tendency in patients is to prefer testing at home without any supervision, as it provides privacy and convenience, coupled with the fact that health tests and medical devices are being technologically designed to overcome this. Some enterprises are working towards creating home use gastrointestinal test panels with prompt results to limit unnecessary hospital visits. This trend is forcing a movement towards less centralized diagnostic solutions which in turn enhances patient uptake and efficiency of the healthcare system.
Gastrointestinal Testing Panel Market Opportunities
- Advancement of Pain-Free Diagnostic Procedures: Non-surgical or ‘non-invasive’ procedures synthesis beliefs for the option of the GI testing panel market that exists in the foreseen future. Since invasive techniques - mostly colonoscopy - have associated disadvantages including risks, discomfort, and financial burden, non-invasive options are preferred. Increasingly, blood tests, stool assays and breath tests are being used to assess gastrointestinal disorders. This trend will propel the market growth as non-invasive options are more convenient and cost-effective in comparison to the conventional methods.
- Partnership with Undewriters: One of the strategic initiatives that can be employed by diagnostic companies to ensure that GI testing panels are widely accepted is to partner with health insurance companies. Incorporating these tests into the insurance packages will enhance the chances of risk factors being identified at an early stage with consequent savings for the healthcare system on expensive management of chronic GI disorders. Such cooperation helps to provide testing for individuals on primary and secondary health insurance and to make gastrointestinal diagnostics services economically viable.
- Research and Development Activities: Targeting Early Detection of Colorectal Cancer The research, development, and marketing of new gadolinium contrast agents for gastrointestinal an breast imaging continue to expand this area of the GI testing market. Advances in genetic tests and blood-based sample liquid biopsies are creating new markets for earlier intervention. The companies focusing on developing the marketing and commercial engine for the benefit of the innovative screening/panel tests’ for din stages colorectal cancer; or pre-cancer lesions stage would expect enormous business potential. All of these measures could contribute to the lowering of mortality figures improving the quality of patients cared for, adding worth to healthcare and the market.
Gastrointestinal Testing Panel Market Regional Insights
The regional trends and factors influencing the Gastrointestinal Testing Panel Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Gastrointestinal Testing Panel Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Gastrointestinal Testing Panel Market
Gastrointestinal Testing Panel Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ XX million |
| Market Size by 2031 | US$ XX Million |
| Global CAGR (2025 - 2031) | 8.3% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Pathogen Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Gastrointestinal Testing Panel Market Players Density: Understanding Its Impact on Business Dynamics
The Gastrointestinal Testing Panel Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Gastrointestinal Testing Panel Market are:
- Becton, Dickinson and Company (BD)
- bioMérieux SA
- DiaSorin S.p.A (Luminex)
- Quidel Corporation
- QIAGEN,
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Gastrointestinal Testing Panel Market top key players overview
Key Selling Points
- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the Gastrointestinal Testing Panel Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the Gastrointestinal Testing Panel Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
This text is related
to segments covered.

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
Bacteria segment, by pathogen type, dominated the market in 2023.
North America region dominated the Gastrointestinal Testing Panel market in 2023.
Advancement of Pain-Free Diagnostic Procedures act as a opportunity for growth of the market in forecast period.
The Gastrointestinal Testing Panel Market is estimated to witness a CAGR of 8.3% from 2023 to 2031
The major factors driving the Gastrointestinal Testing Panel market are:
1. Gastrointestinal Disorders on the Rise
2. Improved Diagnostic Equipment
Players operating in the market are Becton, Dickinson and Company (BD), bioMérieux SA, DiaSorin S.p.A (Luminex), Quidel Corporation, QIAGEN, Seegene, Inc., Savyon Diagnostics, CerTest Biotech S.L., Anatolia Geneworks
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies
1. Becton, Dickinson and Company (BD)
2. bioMérieux SA
3. DiaSorin S.p.A (Luminex)
4. Quidel Corporation
5. QIAGEN,
6. Seegene, Inc.
7. Savyon Diagnostics
8. CerTest Biotech S.L.
9. Anatolia Geneworks
10. ARUP Laboratories
 Get Free Sample For
Get Free Sample For