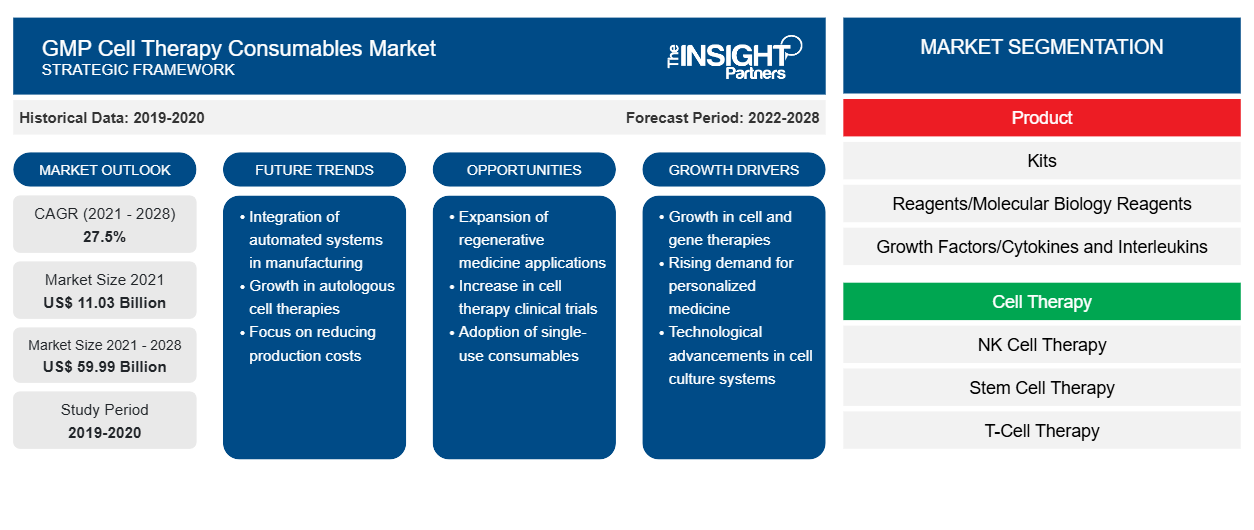
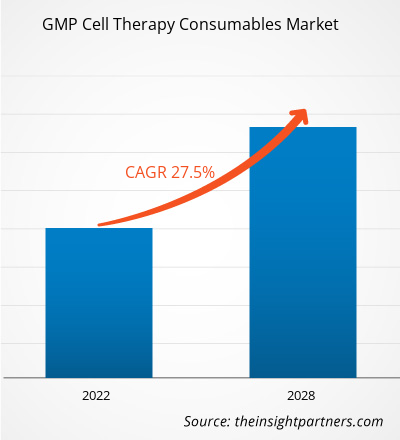
The GMP cell therapy consumables market is expected to grow from US$ 11,031.25 million in 2021 to US$ 59,985.89 million by 2028; it is estimated to grow at a CAGR of 27.5% from 2022 to 2028.
The GMP cell therapy consumables market is segmented on the basis of product, cell therapy, process, end use, and regions. The report offers insights and in-depth analysis of the market, emphasizing various parameters such as dynamics, trends, and opportunities of the market and competitive landscape analysis of leading market players across various regions. It also includes the impact analysis of COVID–19 pandemic across the regions.
Market Insights
Rise in Research & Development and Drug Discovery Drive Market Growth
Drug discovery is a long process that comprises various steps such as early discovery, preclinical phase, clinical phase, and regulatory approval. The process tremendously involves the use of cell therapy consumables to achieve the desired results. According to the World Health Organization, ~10 million deaths due to cancer were recorded in 2020, and the most common cancers are breast, lung, colon, rectum, and prostate cancers. Various research studies have shown positive outcomes of drugs that can effectively treat cancer, neurological disorders, autoimmune disorders, and other chronic disorders. Therefore, the rising prevalence of chronic disorders propels the demand for research and development (R&D) activities.
Moreover, numerous biotechnology companies operating in the market are collaborating with each other to accelerate R&D activities on a global level. For instance, in October 2022, Century Therapeutics and Bristol Myers Squibb announced the research collaboration and license agreement to develop and commercialize up to four induced pluripotent stem cells (“iPSC”) derived, engineered natural killer cell (“iNK”) and/or T cell (“iT”) programs for hematologic malignancies and solid tumors. Additionally, the rising demand for personalized and regenerative medicine has further boosted the growth of the cell therapy consumables market. An increase in drug discovery and R&D requires good manufacturing practices, and GMP consumables are required to obtain desired results from these activities. Thus, an increase in research & development activities, along with a surge in drug discovery, has increased the use of GMP cell therapy consumables.
GMP Cell Therapy Consumables Market -
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
GMP Cell Therapy Consumables Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
GMP Cell Therapy Consumables Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Product-Based Insights
Based on product, the GMP cell therapy consumables market is segmented into kits, reagents/molecular biology reagents, growth factors/cytokines and interleukins, and others. In 2021, the kits segment held the largest market share. However, the growth factors/cytokines and interleukins segment are anticipated to register the highest CAGR in the market during 2022–2028. Reagents and supplements specifically designed for cell culture allow the growth and propagation of a wide spectrum of cell types under controlled conditions. TheraPEAK Products include an array of GMP buffers and reagents dedicated to the creation and development of cell and gene therapies. They are mainly produced in ISO: 13485 plants, such that these GMP solutions for cell and gene therapy are used by various customers in numerous clinical applications. There can be various types of molecular biology reagents that are GMP compliant in nature and mainly used in academics and clinical settings.
Cell Therapy-Based Insights
Based on cell therapy, the GMP cell therapy consumables market is segmented into NK cell therapy, stem cell therapy, T-cell therapy, and others. In 2021, the T-cell therapy segment held the largest share of the market. However, the market in the NK cell therapy segment is expected to grow at the fastest rate during the forecast period. Chimeric antigen receptor (CAR) T-cell therapy is an immunotherapy used to treat certain types of cancer, especially blood cancer or leukemia. The therapy capitalizes the power of immune system protecting against the infections and illnesses by destroying harmful invaders, such as viruses and bacteria. Various research and clinical trials on CAR T-cell therapy are being conducted for cancer treatment. For instance, in January 2022, a new CAR T-cell therapy was developed by University College London (UCL) researchers. Phase-I clinical trials of this therapy carried out at UCL Hospitals (UCLH) concluded that this therapy has fewer toxic effects and is durable. Such increasing clinical trials of CAR T-cell therapy are surging the demand for GMP cell therapy consumables.
Process-Based Insights
Based on process, the GMP cell therapy consumables market is segmented into cell collection and characterization/sorting and separation, cell culture and expansion/preparation, cryopreservation, cell processing and formulation, cell isolation and activation, cell distribution/handling, process monitoring and control/readministration/quality assurance, and others. In 2021, the cell collection and characterization/sorting and separation segment held the largest share of the market. However, the GMP cell therapy consumables market in the cryopreservation segment is expected to grow at the fastest rate during the forecast period. Cell characterization is crucial for understanding the intrinsic factors involved in cellular proliferation, function, and overall process. Cells are sorted and separated based on these characteristics. Companies are building the GMP compliant cell sorter to avoid contamination during the process. For instance, Miltenyi Biotec sells MACSQuant Tyto Cell Sorter, a sterile and GMP compliant multiparametric cell sorting in a closed cartridge system. The company also provides a certificate of analysis (CoA), certificate of origin (CoO), and product information files (PIF) for the regulatory approval of cell manufacturing protocols.
End Use-Based Insights
Based on end use, the GMP cell therapy consumables market is segmented into clinical, commercial, and research. In 2021, the clinical segment held the largest share of the market. Also, the GMP cell therapy consumables market in the same segment is expected to grow at the fastest rate during the forecast period. In clinical use, cell therapy research is carried out on people to evaluate medical, surgical, or behavioral intervention of a particular therapy. Clinical trials are expanding due to the rising prevalence of chronic disorders, , increasing biologics, and rising number of contract research organizations undertaking clinical studies. Various cell therapies are expected to achieve market authorization in the coming years. According to a Nature article, 2,073 active cell therapy agents were in the pipeline in April 2021, which was 572 more than the 2020 update.
The GMP cell therapy consumables market players adopt organic strategies, such as product launch and expansion, to expand their footprint and product portfolio across the world and meet the growing demand. Inorganic growth strategies witnessed in the market are partnerships and collaborations. These growth strategies have allowed the market players to expand their businesses and enhance their geographic presence. Additionally, acquisitions, partnerships, and other growth strategies help strengthen the company’s customer base and increase its product portfolio.
GMP Cell Therapy Consumables Market Regional Insights
The regional trends and factors influencing the GMP Cell Therapy Consumables Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses GMP Cell Therapy Consumables Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for GMP Cell Therapy Consumables Market
GMP Cell Therapy Consumables Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 11.03 Billion |
| Market Size by 2028 | US$ 59.99 Billion |
| Global CAGR (2021 - 2028) | 27.5% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Product
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
GMP Cell Therapy Consumables Market Players Density: Understanding Its Impact on Business Dynamics
The GMP Cell Therapy Consumables Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the GMP Cell Therapy Consumables Market are:
- Sartorius AG
- Thermo Fisher Scientific Inc
- Miltenyi Biotec BV & Co KG
- Bio-Techne Corp
- Corning Inc
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the GMP Cell Therapy Consumables Market top key players overview
- In January 2021, Sartorius signed a strategic collaboration with RoosterBio Inc., a leading supplier of human mesenchymal stem/stromal cell (hMSC). The collaboration advanced the scale-up of hMSC manufacturing for regenerative medicine. RoosterBio and Sartorius created a set of GMP-compatible, customer-centric protocols using RoosterBio’s hMSC and media systems alongside Sartorius’s single-use manufacturing technologies, process control software, and cell analysis tools of hMSC final product manufacturing.
- In September 2020, Bio-Techne Corporation opened an ~61,000 square foot state-of-the-art Good Manufacturing Practices (GMP) manufacturing facility. Located in St. Paul, MN, the new facility is dedicated to support large-scale production of GMP-grade materials, which are an essential component for many immuno-oncology and regenerative medicine cell and gene-modified therapy workflows. This facility helps the company meet current and future demand for the GMP-grade reagents that are necessary to support the rapidly growing cell therapy market.
GMP Cell Therapy Consumables Market - Company Profiles
- Sartorius AG
- Thermo Fisher Scientific Inc
- Miltenyi Biotec BV & Co KG
- Bio-Techne Corp
- Corning Inc
- FUJIFILM Irvine Scientific Inc
- Lonza Group AG
- BPS Bioscience Inc
- Merck KGaA
- Global Life Sciences Solutions USA LLC.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset


- Environmental Consulting Service Market
- Vertical Farming Crops Market
- Trade Promotion Management Software Market
- Advanced Planning and Scheduling Software Market
- Batter and Breader Premixes Market
- Hummus Market
- Ketogenic Diet Market
- Digital Language Learning Market
- Saudi Arabia Drywall Panels Market
- Pharmacovigilance and Drug Safety Software Market

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Product, Cell Therapy, Process, and End Use

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, Saudi Arabia, South Africa, South Korea, Spain, United Arab Emirates, United Kingdom, United States
Frequently Asked Questions
The global GMP cell therapy consumables based on regions is segmented into North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America. In 2021, the North American area held the largest market share. However, the Asia Pacific region is estimated to grow at the fastest CAGR of 29.6% during the forecast period
The global GMP cell therapy consumables based on the product are segmented into kits, reagents/molecular biology reagents, cell culture media and supplements, growth factors/cytokines and interleukins (including protein and nucleic acid purification buffers), and others. The kits segment held the largest market share in 2021. However, growth factors/cytokines and interleukins is anticipated to register the highest CAGR during the forecast period.
Stringent regulatory policies are expected to restrict the GMP cell therapy consumables growth during the forecast period.
The GMP cell therapy consumables majorly consist of the players, such as Sartorius AG, Thermo Fisher Scientific Inc, Miltenyi Biotec BV & Co KG, Bio-Techne Corp, Corning Inc, FUJIFILM Irvine Scientific Inc, Lonza Group AG, BPS Bioscience Inc, Merck KGaA, and Global Life Sciences Solutions USA LLC among others.
The factors driving the growth of GMP cell therapy consumables are Increase in research & development and drug discovery, as well as increase in strategic collaborations.
Good Manufacturing Practices (GMP) is a system for ensuring that products are consistently produced and controlled according to quality standards. Cell therapy manufacturing processes range in complexity. Cell therapy manufacturing processes require an underpinning pharmaceutical quality system and quality control (QC) laboratory, and all aspects of cell therapy manufacturing require trained personnel. As the process is complex there may be chances of contamination, therefore GMP in cell therapy is becoming mandatory. Also, the use of cell therapy for treatment of various diseases is increasing the demand for cell therapy consumables that are manufactured according to GMP regulations.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - GMP Cell Therapy Consumables Market
- Sartorius AG
- Thermo Fisher Scientific Inc
- Miltenyi Biotec BV & Co KG
- Bio-Techne Corp
- Corning Inc
- FUJIFILM Irvine Scientific Inc
- Lonza Group AG
- BPS Bioscience Inc
- Merck KGaA
- Global Life Sciences Solutions USA LLC
 Get Free Sample For
Get Free Sample For