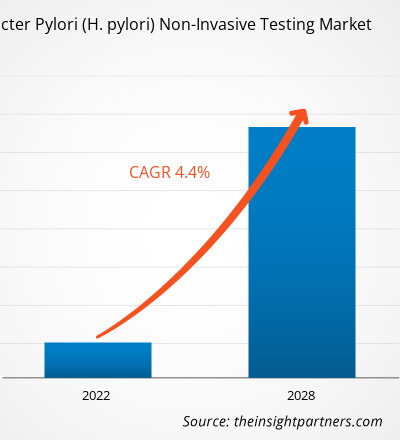
The Helicobacter Pylori (H. pylori) non-invasive testing market is expected to grow from US$ 596.82 million in 2021 to US$ 800.04 million by 2028; it is estimated to grow at a CAGR of 4.4% from 2022 to 2028.
The rising prevalence of H. pylori infection and technological advancements are driving the overall market growth. Further, strategies implemented by major players provide lucrative opportunities in the global market.
Helicobacter Pylori (HP) is a major factor responsible for peptic ulcer disease and gastritis. As per the Public Library of Science (PLOS) report, approximately one-third of all adults in Northern Europe and North America are infected with HP, with a very high prevalence in Africa, South & Central America, Asia, and Southern & Eastern Europe accounting for 50%. Also, HP infection is high among adults with some geographical variations. For example, the National Institute of Health (NIH) report states that the infection of H. pylori among adolescents is 30-50%, and among adults, it reaches 90% in developing countries.
The Clinical and Experimental Pediatrics (CEP) report states that the prevalence of H. pylori infection has decreased. Although the H. pylori infection rate has decreased in South Korea and developed countries, 33% of asymptomatic or healthy children are still infected serologically worldwide, as per the same report. For example, developed countries such as Japan, Germany, the Netherlands, and the US reported low seroprevalence, accounting for 7.7%, 11.8%, 9.8%, and 5.7%, respectively, in 2020. However, high infection rates of 27.2%, 65.9%, 25.8%, and 40.4% were reported in Chile, Venezuela, Iran, and Nigeria, respectively, in the same year. Therefore, the rising prevalence of H. pylori infection accelerates the demand for non-invasive testing, ultimately fueling the growth of the Helicobacter Pylori (H. pylori) non-invasive testing market during the forecast period.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Helicobacter Pylori (H. pylori) Non-Invasive Testing Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Helicobacter Pylori (H. pylori) Non-Invasive Testing Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Helicobacter Pylori (H. pylori) is a bacterial infection that is majorly detected in patients suffering from gastric and duodenal ulcers. For instance, the Centers for Disease Control and Infection (CDC) report states that the prevalence of H. pylori infection worldwide is ~50% and ~80-90% in developing countries. Moreover, the prevalence of H. pylori is estimated to be 35-40% in the US. The high prevalence of H. pylori infection positively influences the adoption of non-invasive diagnostic solutions. Technological advancements and innovations in the H. pylori non-invasive testing solutions offer accurate results and greater patient satisfaction among patients suffering from bacterial infection. For example, point-of-care testing (POCT) provides accurate results at an affordable price in a short time. As people with multiple health conditions are usually more vulnerable to infections and seek early detection of H. pylori infection, greater accessibility of POCT and easy availability of numerous invasive and non-invasive devices positively impact the market growth. For instance, in May 2022, Biomerica, Inc. announced a new product launch for the CE mark for its "hp+detect'" diagnostic test responsible for detecting H. pylori bacteria. The new product "hp+detect" product is responsible for detecting H. pylori bacteria, having a prevalence of 35% among the US population and 45% of Europe's five largest countries.
The low diagnosis rate acts as a major restraint for the Helicobacter pylori (H. pylori) non-invasive testing market. Low socio-economic status or a low level of education are associated with an increased prevalence of H. pylori infection. In Australia, the pooled H. pylori prevalence estimated for the general population was 24.6%, but it recorded 76.0% prevalence in the rural Western Australian indigenous community. In the US, the pooled Helicobacter pylori prevalence estimated in the general population was 35.6%; however, in the Alaskan indigenous population, it was 74.8%. Helicobacter pylori (H. pylori) infection is high because it remains asymptomatic for an extended period, so the rate of diagnosis is low for this infection. Most people with H. pylori infection are asymptomatic. This issue can delay the diagnosis of infection, which will limit the demand for non-invasive test kits.
Helicobacter Pylori (H. pylori) Non-Invasive Testing Market - Regional Overview
China holds a considerable share in the Asia Pacific Helicobacter pylori (H. pylori) non-invasive testing market. China is a developing country in Asia Pacific with a well-established healthcare system and a fast-growing pharmaceutical industry. China is home to various medical, pharmaceutical, and biotechnology product manufacturing companies operating in the global market. These pharmaceuticals and biotechnology companies are involved in the development of various testing kits and devices for H. pylori detection owing to the high prevalence of various H. pylori infections and gastric (stomach) cancer cases, which boosts the growth of the market.
Japan is one of the leading countries with the most advanced medical industry. According to Roswell Park Comprehensive Cancer Center, Japan experiences disproportionately high rates of stomach cancer cases due to high rates of infection with H. pylori and high consumption of smoked and salted foods. Also, gastric cancer is the third deadliest cancer, resulting in the death of ~50,000 people each year in Japan. Furthermore, as per a study (2020) published in Wiley Online Library, H. pylori causes ~98% of gastric cancer in Japan. Although there were many reported reasons for gastric cancer cases, recent studies have shown that the majority of gastric cancer incidence is due to the infection caused by H. pylori. The aforementioned factors are expected to positively impact the Helicobacter pylori (H. pylori) non-invasive testing market growth in Japan during the forecast period.
Helicobacter Pylori (H. pylori) Non-Invasive Testing Market - Test Type Based Insights
Based on test type, the Helicobacter Pylori (H. pylori) non-invasive testing market is segmented into serology, stool antigen, and urea breath. The urea breath segment is estimated to hold the largest market share and the highest CAGR from 2022 to 2028. According to the National Institute of Health (NIH) report, non-invasive H. pylori diagnostic tests are available for point-of-care (POC) intended for primary care by involving IgG serology, C-urea breath test (UBT), and monoclonal stool antigen. Among these, only UBT provides accuracy in confirming current infection or eradication. The Gut and Liver report shows that UBT is non-invasive and accurate with safety among pediatric patients and pregnant women. Thus, the aforementioned factors are likely to boost the market growth for this segment from 2022 to 2028.
Companies operating in the Helicobacter Pylori (H. pylori) non-invasive testing market adopt the product innovation strategy to meet the evolving customer demands across the world, which also permits them to maintain their brand name in the market.
Helicobacter Pylori (H. pylori) Non-Invasive Testing Market – Segmentation
Based on test type, the Helicobacter Pylori (H. pylori) non-invasive testing market is segmented into serology, stool antigen, and urea breath. The urea breath segment accounted for the largest market share in 2021 and is expected to register the highest CAGR from 2022 to 2028. Based on test method, the market is bifurcated into laboratory based and point-of-care. The laboratory based segment led the market in 2021 and is expected to retain its dominance during the forecast period. Based on end user, the market is segmented into hospitals, clinics, and diagnostic laboratories. The hospitals segment led the market in 2021 and is expected to retain its dominance during the forecast period. Based on geography, the market is primarily segmented into North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America.
Helicobacter Pylori Non-invasive Testing Helicobacter Pylori (H. pylori) Non-Invasive Testing Market Regional Insights
Helicobacter Pylori (H. pylori) Non-Invasive Testing Market Regional Insights
The regional trends and factors influencing the Helicobacter Pylori (H. pylori) Non-Invasive Testing Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Helicobacter Pylori (H. pylori) Non-Invasive Testing Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Helicobacter Pylori (H. pylori) Non-Invasive Testing Market
Helicobacter Pylori (H. pylori) Non-Invasive Testing Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 596.82 Million |
| Market Size by 2028 | US$ 800.04 Million |
| Global CAGR (2021 - 2028) | 4.4% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Test Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Helicobacter Pylori (H. pylori) Non-Invasive Testing Market Players Density: Understanding Its Impact on Business Dynamics
The Helicobacter Pylori (H. pylori) Non-Invasive Testing Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Helicobacter Pylori (H. pylori) Non-Invasive Testing Market are:
- DiaSorin S.p.A.
- Meridian Bioscience Inc.
- QuidelOrtho Corporation
- Abbott Laboratories
- CerTest Biotec
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Helicobacter Pylori (H. pylori) Non-Invasive Testing Market top key players overview
Helicobacter Pylori (H. pylori) Non-Invasive Testing Market - Company Profiles
- DiaSorin S.p.A,
- Meridian Bioscience, Inc.,
- QuidelOrtho Corporation,
- Abbott Laboratories,
- Thermo Fischer Scientific,
- CerTest Biotec,
- Sekisui Diagnostics,
- Coris BioConcept,
- Shenzhen Zhonghe Headway Bio-Sci & Tech Co., Ltd.,
- Bio-Rad Laboratories, Inc.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Test Type, Test Method, and End User

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, RoE, RoMEA, RoSCAM, Saudi Arabia, South Africa, South Korea, Spain, United Arab Emirates, United Kingdom, United States
Frequently Asked Questions
Global Helicobacter pylori (H. pylori) non-invasive testing market is segmented by region into North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America. In North America, the U.S. is the largest market for H. pylori non-invasive testing market. The growing prevalence of stomach cancer, duodenal ulcers, and gastric ulcers and the development of health care facilities will drive H. pylori non-invasive testing market growth in North America. Further, the rising geriatric population, high prevalence of H. pylori, gastric cancer cases, and expanding partnerships among key players are the key factor responsible for the Asia-Pacific regional growth for H. pylori non-invasive testing market accounting fastest growth of the region during the coming years.
DiaSorin S.p.A., Meridian Bioscience, QuidelOrtho Corporation, Abbott, Thermo Fisher Scientific, Inc., Certest Biotec S.L., Sekisui Diagnostics, CorisBioconcept SPRL, Shenzhen Zhonghe Headway Bio-Sci & Tech Co., Ltd., and Bio-Rad Laboratories, Inc. are among the leading companies operating in the H. pylori non-invasive testing market.
The diagnostic laboratories segment dominated the global Helicobacter pylori (H. pylori) non-invasive testing market and accounted for the largest market share of 59.01% in 2021.
Based on test type, the urea breath segment took the forefront leaders in the worldwide market by accounting largest share in 2021 and is expected to continue to do so till the forecast period.
Based on the test method, laboratory-based test method segment took the forefront leaders in the worldwide market by accounting largest share in 2021 and is expected to continue to do so till the forecast period.
Helicobacter pylori (H. pylori) is a very motile, spiral, or curved rod-shaped Gram-negative bacteria comprising multiple flagella that live in the gastric mucous-coated lining and the gastric pits of the epithelial tissue of the stomach and/or duodenum. Currently, non-invasive tests are the first methods for diagnosing H. Pylori infection. These tests include serological tests, stool antigen research, and urea breath test.
Increasing prevalence of H. pylori bacterial infection and new product launches and FDA approvals are the most significant factors responsible for the overall market growth.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Helicobacter Pylori (H. pylori) Non-invasive Testing Market
- DiaSorin S.p.A.
- Meridian Bioscience Inc.
- QuidelOrtho Corporation
- Abbott Laboratories
- CerTest Biotec
- Thermo Fisher Scientific Inc.
- Sekisui Diagnostics
- Coris BioConcept
- Shenzhen Zhonghe Headway Bio-Sci & Tech Co., Ltd
- Bio-Rad Laboratories Inc.
 Get Free Sample For
Get Free Sample For