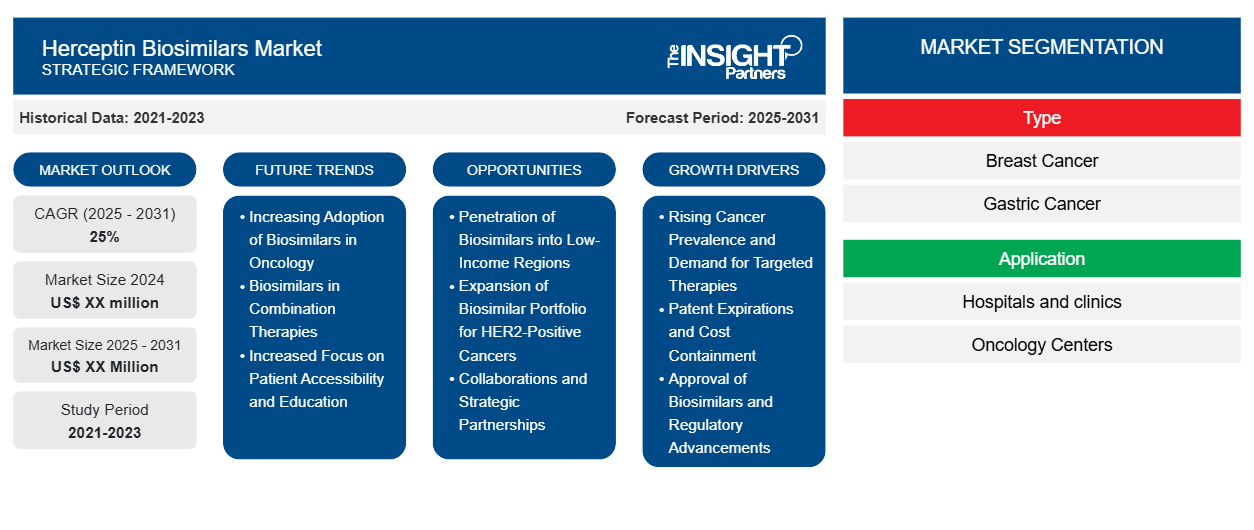
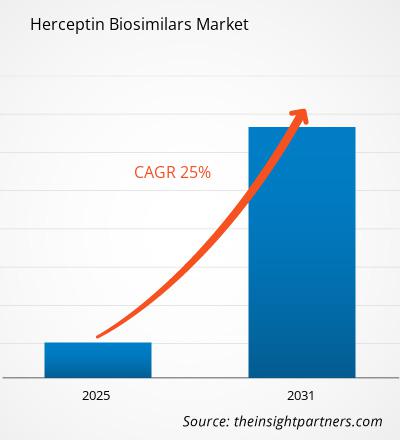
The Herceptin Biosimilars Market is expected to register a CAGR of 25% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The report is segmented by Type (Breast Cancer, Gastric Cancer, Others.); and Application (Hospitals and clinics, Oncology Centers, Others). The global analysis is broken down at the regional level and for major countries. The market evaluation is presented in US$ for the above segmental analysis.
Purpose of the Report
The report Herceptin Biosimilars Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
Herceptin Biosimilars Market Segmentation
Type
- Breast Cancer
- Gastric Cancer
Application
- Hospitals and clinics
- Oncology Centers
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Herceptin Biosimilars Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Herceptin Biosimilars Market Growth Drivers
- Rising Cancer Prevalence and Demand for Targeted Therapies: The Herceptin biosimilars market is significantly driven by the increasing prevalence of HER2-positive breast cancer. HER2-positive breast cancer is a subtype of breast cancer where the cancer cells produce excess HER2 proteins, leading to aggressive tumor growth. Trastuzumab, branded as Herceptin, is a targeted therapy that has been proven effective in treating HER2-positive breast cancer. As the incidence of breast cancer rises globally, especially in developing countries, the demand for cost-effective treatments like Herceptin biosimilars has grown substantially. Biosimilars of Herceptin, which are nearly identical versions of the original biologic drug, are becoming popular because they offer the same therapeutic benefits at a significantly lower cost. This allows patients, particularly in lower-income regions, to access critical treatments. Furthermore, the success of Herceptin in treating HER2-positive breast cancer has led to its approval for other HER2-positive cancers, such as gastric cancer. This broader application of Herceptin continues to fuel the market for both the original biologic and its biosimilars. The shift toward precision oncology, where drugs are tailored to target specific genetic mutations or receptors like HER2, is another factor contributing to the increased demand for targeted therapies. As the cancer burden continues to grow, Herceptin biosimilars are expected to gain more prominence due to their affordability, improved accessibility, and effective treatment outcomes.
- Patent Expirations and Cost Containment: The expiration of patents for Herceptin, the reference biologic, is one of the primary growth drivers for the Herceptin biosimilar market. As patents for Herceptin expired in multiple regions, it created a market opportunity for the development and approval of biosimilars, which are less expensive alternatives that replicate the biological product’s safety, efficacy, and quality. With healthcare costs rising globally, especially for high-cost treatments like biologic drugs, biosimilars offer a more affordable solution without compromising patient care. For healthcare systems, especially in countries with stringent cost-containment measures, Herceptin biosimilars present a promising way to reduce overall cancer treatment expenses. Biosimilars often come at a lower price, enabling hospitals, clinics, and insurers to provide life-saving treatments to a larger number of patients, helping to address the issue of accessibility. Additionally, as healthcare systems adopt policies that encourage the use of biosimilars in place of their reference biologics, the demand for Herceptin biosimilars will continue to rise. The ongoing efforts to make cancer treatments more cost-effective globally, alongside initiatives such as biosimilar substitution policies, are expected to create a strong market demand for biosimilars in the years to come. This increasing reliance on biosimilars to mitigate the high costs of cancer therapies ensures that Herceptin biosimilars will maintain a strong market position.
- Approval of Biosimilars and Regulatory Advancements: The Herceptin biosimilars market has witnessed rapid growth due to the approval of several biosimilar versions of trastuzumab by major regulatory bodies, including the U.S. FDA and the European Medicines Agency (EMA). As biosimilar approval processes have become more streamlined and well-defined, more pharmaceutical companies have entered the biosimilar market, contributing to increased competition and affordability. Regulatory advancements have enhanced confidence in the safety and efficacy of biosimilars, which has played a crucial role in driving their adoption. Unlike generic drugs, biosimilars undergo a comprehensive approval process that includes rigorous clinical trials to demonstrate their equivalence to the reference biologic, ensuring that patients receive the same therapeutic benefits. These approvals have reassured healthcare providers, allowing them to prescribe biosimilars confidently. In addition, regulatory agencies have introduced pathways to facilitate faster approval timelines, reducing the barriers to market entry for biosimilars. The increasing acceptance of biosimilars in established markets like North America and Europe, along with the continued evolution of global regulatory frameworks, is expected to further enhance the growth prospects for Herceptin biosimilars. With biosimilars continuing to gain approval for additional indications beyond breast cancer, including gastric cancer and other HER2-positive cancers, the future of Herceptin biosimilars looks promising.
Herceptin Biosimilars Market Future Trends
- Increasing Adoption of Biosimilars in Oncology: The increasing adoption of biosimilars in oncology is one of the most prominent trends in the Herceptin biosimilars market. Over the past few years, as more data has become available regarding the safety and efficacy of biosimilars, healthcare providers are increasingly comfortable with incorporating them into cancer treatment regimens. The positive clinical outcomes observed with biosimilars such as trastuzumab have bolstered their credibility in the oncology field. As oncologists and cancer centers become more familiar with biosimilars, their use is expected to grow. Additionally, the cost-effectiveness of biosimilars compared to the original biologic drug is appealing to healthcare systems, especially in regions with limited resources. The demand for Herceptin biosimilars is also expected to increase as patients seek more affordable treatment options. Hospitals and insurers are more likely to favor biosimilars, not only for their lower cost but also due to the growing evidence of their comparable effectiveness to the reference biologic. As cancer treatment paradigms continue to shift toward precision medicine, the role of biosimilars, including Herceptin biosimilars, will expand as part of the broader strategy to provide affordable and effective cancer care. This trend is likely to gain momentum in both developed and emerging markets, where there is a push to make high-cost cancer treatments accessible to a wider patient population.
- Biosimilars in Combination Therapies: A key future trend in the Herceptin biosimilars market is the increasing use of biosimilars in combination therapies. Combination therapies, where multiple treatment modalities are used together to fight cancer, have become a cornerstone of modern oncology. In the case of HER2-positive cancers, biosimilars such as Herceptin biosimilars are increasingly being used in combination with other drugs, such as chemotherapy agents and immune checkpoint inhibitors, to enhance therapeutic efficacy. The benefits of combining Herceptin biosimilars with other cancer therapies include improved overall survival rates and reduced risk of relapse. Researchers are also exploring the potential of combining biosimilars with novel targeted therapies, offering the possibility of synergistic effects. This trend is likely to continue, as combination therapies have become an essential approach for treating complex cancers that have multiple pathways for growth. As biosimilars like Herceptin are used in more combination regimens, the demand for these drugs will continue to grow, providing greater treatment options for patients. In addition, biosimilar manufacturers are expected to invest in developing combination therapies that are both effective and affordable, which would further drive the adoption of Herceptin biosimilars in oncology practices.
- Increased Focus on Patient Accessibility and Education: As the Herceptin biosimilar market matures, there will be an increased focus on improving patient accessibility and education. Despite the proven efficacy and affordability of biosimilars, patient and healthcare provider hesitation remains a barrier to their widespread adoption. To address this, both manufacturers and healthcare organizations are focusing on educational initiatives to inform patients and healthcare providers about the benefits of biosimilars. These programs aim to clarify misconceptions about biosimilars, emphasizing that they are safe, effective alternatives to the original biologic. Furthermore, the focus on patient accessibility will become increasingly important in markets where cancer treatment options are limited, and access to branded biologics is not feasible. By improving awareness and promoting the cost-effectiveness of biosimilars, healthcare providers will be more likely to incorporate these treatments into their protocols. As governments and insurance companies also work to support the coverage of biosimilars, patient access to these treatments is expected to improve. Educational campaigns and increased accessibility to Herceptin biosimilars will play a crucial role in driving broader market adoption, particularly in regions with a high unmet need for affordable cancer therapies.
Herceptin Biosimilars Market Opportunities
- Penetration of Biosimilars into Low-Income Regions: One of the most significant growth opportunities for the Herceptin biosimilars market lies in the penetration of low-income regions, where access to expensive biologic drugs like Herceptin is often limited. In many developing countries, the high cost of branded biologics poses a significant barrier to cancer treatment, leaving many patients without access to the therapies they need. However, the affordability of Herceptin biosimilars presents a compelling solution to this problem. Biosimilars offer a more accessible, cost-effective alternative while maintaining the same efficacy and safety as the reference biologic. As governments and healthcare organizations in low-income regions continue to improve cancer care infrastructure and expand access to essential medicines, Herceptin biosimilars will play a key role in making life-saving treatments more widely available. By focusing on pricing strategies and partnerships with local governments and non-governmental organizations (NGOs), biosimilar manufacturers can tap into the growing demand for affordable cancer treatments in these regions. This opportunity is especially critical in countries where cancer incidence is rising, and healthcare systems are struggling to meet the increasing demand for cancer care.
- Expansion of Biosimilar Portfolio for HER2-Positive Cancers: A major growth opportunity for the Herceptin biosimilars market lies in the expansion of the biosimilar portfolio for other HER2-positive cancers beyond breast cancer, including gastric cancer, endometrial cancer, and other HER2-driven cancers. While the primary indication for Herceptin biosimilars has been HER2-positive breast cancer, research is expanding into other cancers that also overexpress the HER2 receptor. The ability to treat these additional cancer types with Herceptin biosimilars offers a significant opportunity for manufacturers to broaden their market reach and increase sales. Manufacturers that develop and market Herceptin biosimilars for these additional HER2-positive indications will gain a competitive advantage and expand their market share. With a growing body of clinical evidence supporting the efficacy of Herceptin in treating various cancers, the future looks promising for expanding the role of Herceptin biosimilars in oncology.
- Collaborations and Strategic Partnerships: Strategic collaborations and partnerships between biosimilar manufacturers and healthcare providers offer significant opportunities for expanding market share in the Herceptin biosimilars market. By collaborating with hospitals, oncology centers, and healthcare systems, biosimilar manufacturers can ensure better distribution of their products, streamline the approval process, and increase visibility in the oncology community. Additionally, partnerships with governments and health organizations in emerging markets are crucial for improving access to affordable biosimilars. Such collaborations can help reduce the financial burden of cancer treatment for patients, especially in regions where cancer care is underfunded or inaccessible. Through joint ventures and licensing agreements, biosimilar manufacturers can leverage the expertise and infrastructure of healthcare providers to increase adoption and market penetration of Herceptin biosimilars, creating new growth opportunities.
Herceptin Biosimilars Market Regional Insights
The regional trends and factors influencing the Herceptin Biosimilars Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Herceptin Biosimilars Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
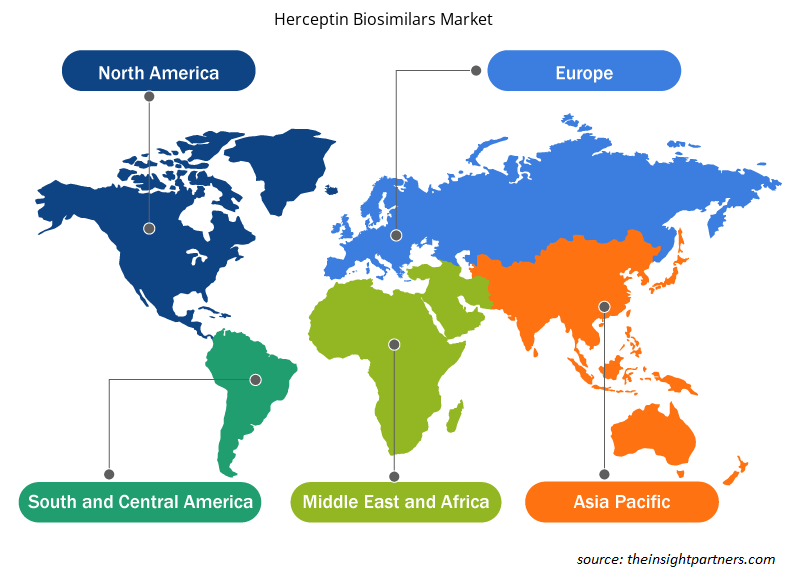
- Get the Regional Specific Data for Herceptin Biosimilars Market
Herceptin Biosimilars Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ XX million |
| Market Size by 2031 | US$ XX Million |
| Global CAGR (2025 - 2031) | 25% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
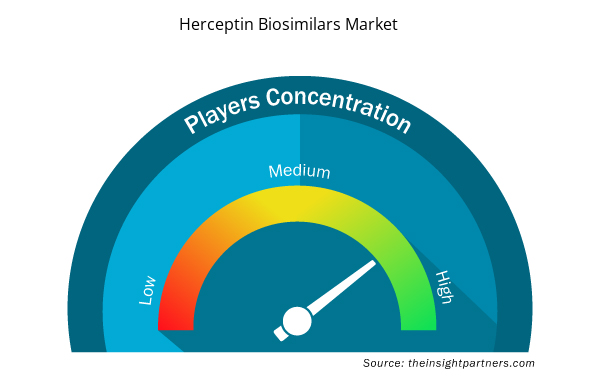
Herceptin Biosimilars Market Players Density: Understanding Its Impact on Business Dynamics
The Herceptin Biosimilars Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Herceptin Biosimilars Market are:
- Amgen Inc.
- AryoGen Biopharma
- Roche Holding AG
- Samsung bioepis Co,.Ltd.
- Biocon Limited
- Celltrion Inc.
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Herceptin Biosimilars Market top key players overview
Key Selling Points
- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the Herceptin Biosimilars Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the Herceptin Biosimilars Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
This text is related
to segments covered.

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
The Herceptin Biosimilars Market is estimated to witness a CAGR of 25% from 2025 to 2031.
The major factors driving the Herceptin Biosimilars Market are Rising Cancer Prevalence and Demand for Targeted Therapies, Patent Expirations and Cost Containment, and Approval of Biosimilars and Regulatory Advancements.
Future trends in the Herceptin Biosimilars Market are - Increasing Adoption of Biosimilars in Oncology, Biosimilars in Combination Therapies, Increased Focus on Patient Accessibility and Education.
Some of the players operating in the market are Amgen Inc., AryoGen Biopharma, Roche Holding AG, Samsung bioepis Co,.Ltd., Biocon Limited, Celltrion Inc., Pfizer Inc., Merck & Co., Inc., Gedeon Richter Plc, Genoa Biopharma Company Ltd
The report can be delivered in PDF/PPT format; we can also share an excel datasheet based on the request.
Some customization options available based on the request are an additional 3–5 company profiles and a country-specific analysis of 3–5 countries of your choice. Customizations are to be requested/discussed before making final order confirmation# as our team would review the same and check the feasibility.
Trends and growth analysis reports related to Life Sciences : READ MORE..
1. Amgen Inc.
2. AryoGen Biopharma
3. Roche Holding AG
4. Samsung bioepis Co,.Ltd.
5. Biocon Limited
6. Celltrion Inc.
7. Pfizer Inc.
8. Merck and Co., Inc.
9. Gedeon Richter Plc
10. Genor Biopharma Company Ltd
 Get Free Sample For
Get Free Sample For