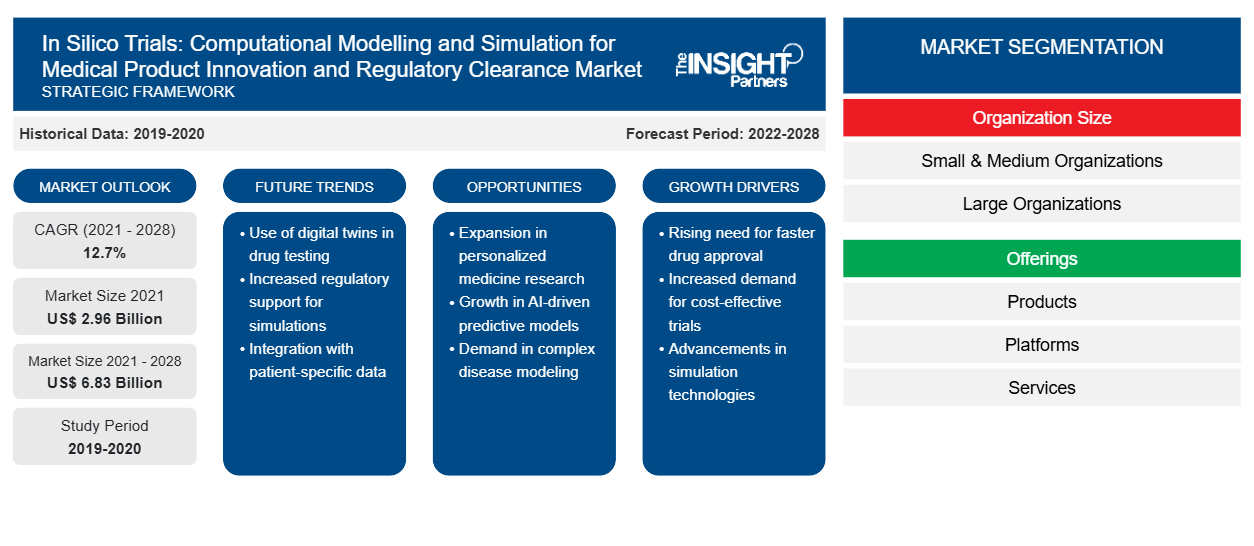
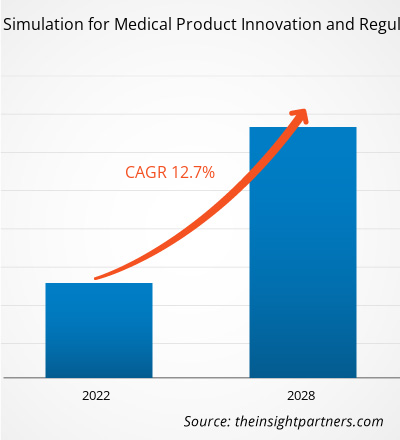
The in silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is projected to reach US$ 6,830.99 million in 2028 from US$ 2,957.65 million in 2021; it is estimated to grow at a CAGR of 12.7% from 2021 to 2028.
In silico clinical trials refer to developing patient-specific models to form virtual cohorts for testing the safety and efficacy of new drugs and medical devices. Companies use sophisticated computational modeling and simulation techniques to test their drug candidates in virtual patients before trying them in humans. In silico modeling, also known as computer modeling, allows researchers to simulate behaviors on a computer screen.
The in silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented on the basis of organization size, offering, application, clinical indication, end user, and geography. The market, based on geography, is broadly segmented into North America, Europe, Asia Pacific, the Middle East & Africa, and South & Central America. The report offers insights and a comprehensive analysis of the market, emphasizing parameters such as in silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market size, technological advancements, and market dynamics, along with the analysis of the competitive landscape of the globally leading market players.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Market Insights
Rising Concerns Over Animal Welfare to Drive In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Growth during Forecast Period
The rapid use of animals in clinical trials is mainly due to the biological similarities between animals and humans. Animals provide adequate human biology and diseases models to yield relevant information, and consequently, animal models offers significant human health benefits. However, using animals for clinical studies is not the only way of drug and devices discovery, as almost every clinical study causes harm to the animal and its progeny in any manner. For instance, in January 2020, the U.S. Department of Agriculture had reported that approximately 300,000 animals were involved in pain-causing experimental activities in just one year.
Moreover, as mentioned by People for the Ethical Treatment of Animals (PETA), each year, more than 100 million animals, including mice, frogs, rabbits, hamsters, and guinea pigs, are killed in US laboratories. The purpose of sacrificing these animals is biology lessons, medical training, curiosity-driven experimentation, and chemical, drug, food, and cosmetics testing. In silico clinical trials offer an effective way of replacing animal anatomies for conducting various experiments and research & development activities, thus, supporting the growth of the in silico trials market. Additionally, rapidly increasing concerns over animal welfare and consistent efforts of human and animal rights and welfare organizations are boosting the global in silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market growth.
Organization Size Insights
Based on organization size, the global in silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is bifurcated into small & medium organizations and large organizations. In 2021, the large organizations segment held a larger market share. Moreover, the small and medium organization segment is expected to register the highest CAGR in the market during 2021–2028 due to the high usage of the in silico trial methods in large research organizations and institutes. However, in the recent era, medium-sized pharmaceutical and biopharmaceutical companies are adopting the in silico model processes to reach their trial processes and increase their efficacy in creating new products.
Offering Insights
Based on offering, the global in silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented into products, platforms, and services. In 2021, the products segment held the largest market share. Moreover, the services segment is expected to register the highest CAGR in the market during 2021–2028. The factor attributing to the growth of this segment is low-risk, cost-effective, and virtual environment in in silico trials computational modelling and simulation technology.
Application Insights
Based on application, the global in silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented into product design and discovery, product development, pre-clinical targeting, assessment of drugs & other biomedical products, and others. In 2021, the product design and discovery segment held the largest market share. Moreover, the pre-clinical targeting segment is expected to register the highest CAGR in the market during 2021–2028. The factor attributing the growth of this segment is the incorporation of advanced technologies such as machine learning, and artificial intelligence is applied for drug discovery and design applications.
Clinical Indication Insights
Based on clinical indication, the global in silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented into cardiovascular diseases, neurodegenerative diseases, oncology, rare diseases, metabolic diseases, immune based diseases, infectious diseases, and others. In 2021, the cardiovascular diseases segment held the largest in silico trials market share. Moreover, the infectious diseases segment is expected to register the highest CAGR in the market during 2021–2028. The factor attributing to the growth of this segment is the rising COVID-19 cases across the globe and strong involvement of the immune system modelling to cure the disease.
End-User Insights
Based on end-users, the global in silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented into pharmaceutical and biopharmaceutical companies, medical technology companies, contract research organizations, and others. In 2021, the pharmaceutical and biopharmaceutical companies segment held the largest in silico trials market share. Moreover, the contract research organization segment is expected to register the highest CAGR in the market during 2021–2028.
Product launches, mergers & acquisitions, and collaborations are highly adopted strategies by the global market players. A few of the recent key market developments are listed below:
- In September 2021, Sensyne Health announced that it had launched SENSIGHT, an AI-enabled global data analytics platform for the healthcare and life sciences sectors.
- In February 2022, In SilicoTrials announced that it partnered with IonsGate Preclinical Services Inc (IonsGate) to leverage innovative technology like Modeling and Simulation.
Though the COVID-19 pandemic crisis had a devastating effect on several industries, the in-silico trial market experienced significant growth during this period due to the computer-aided drug discovery method. The demand for in silico trials with the help of computational modeling has witnessed a higher growth rate due to increased R&D activities among researchers and biotechnological and biopharmaceutical companies to limit the spread of the coronavirus disease. The increasing mutation rate of the COVID-19 pandemic has encouraged scientists to discover an effective treatment against the disease. Thus, the significance of research and drug discovery was raised, thereby driving the in silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market growth. The above-stated factors show that the pandemic generated substantial investment opportunities in the market.
In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Segmentation
The global In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance market is segmented on the basis of organization size, offering, application, clinical indication, end user, and geography. Based on organization size, the In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance market is bifurcated into small & medium organizations and large organizations. Based on offering, the In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance market is segmented into products, platforms, and services.
Based on application, the In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance market is segmented into product design and discovery, product development, pre-clinical targeting, assessment of drugs and other biomedical products, and others.
Based on clinical indication, the In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance market is segmented into cardiovascular diseases, neurodegenerative diseases, oncology, rare diseases, metabolic diseases, immune based diseases, infectious diseases, and others.
Based on end-users, the In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance market is segmented into pharmaceutical and biopharmaceutical companies, medical technology companies, contract research organizations, and others.
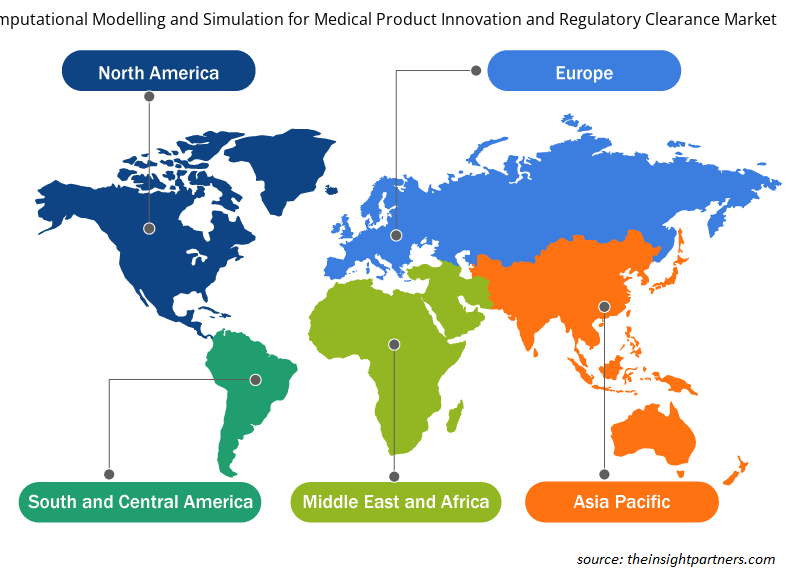
The In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance market, based on geography, is broadly segmented into North America, Europe, Asia Pacific, the Middle East & Africa, and South and Central America. The market in North America is further segmented into the US, Canada, and Mexico. The European market is segmented into France, Germany, the UK, Spain, Italy, and the Rest of Europe.
In Silico Clinical Trials In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Regional Insights
The regional trends and factors influencing the In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market
In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 2.96 Billion |
| Market Size by 2028 | US$ 6.83 Billion |
| Global CAGR (2021 - 2028) | 12.7% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Organization Size
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Players Density: Understanding Its Impact on Business Dynamics
The In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market are:
- InSilico Trials Technologies
- Feops
- CADFEM Medical GmbH
- Dassault Systèmes
- Virtonomy GmbH
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market top key players overview
The market in Asia Pacific is segmented into China, India, Japan, Australia, South Korea, and the Rest of APAC. The market in the MEA is further segmented into Saudi Arabia, the UAE, South Africa, and the Rest of the MEA. The market in South and Central America is segmented into Brazil, Argentina, and the Rest of South and Central America. InSilico Trials Technologies; FEops; CADFEM Medical GmbH; Dassault Systemes SE; Virtonomy GmbH; Certara Inc.; Computational Life; Novadiscovery; TwInsight Medical; Ansys, Inc.; Synopsys, Inc.; Sensyne Health plc; Phesi; Tempus; and Cerner Corporation are among the leading companies operating in the global market during the forecast period of 2021 to 2028. In May 2020, UK-based Exscientia raised US$60 million in a series C financing round led by Novo Holdings, the wholly-owned holding company of Danish diabetes medicine maker Novo Nordisk, along with German drug development company Evotec, US pharmaceutical company Bristol Myers Squibb, and Asian-based private investment partnership GT Healthcare Capital. This brought the company’s total funding to just over US$100 million. The new capital will be used in part to expand the company’s AI capabilities in biology.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset


- GNSS Chip Market
- Hand Sanitizer Market
- Quantitative Structure-Activity Relationship (QSAR) Market
- Human Microbiome Market
- Greens Powder Market
- Point of Care Diagnostics Market
- Cosmetic Bioactive Ingredients Market
- Latent TB Detection Market
- Electronic Data Interchange Market
- Enteral Nutrition Market

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Organization Size, Offerings, Application, Clinical Indication, and End User

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, Saudi Arabia, South Africa, South Korea, Spain, United Arab Emirates, United Kingdom, United States
Frequently Asked Questions
Global in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented by region into North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America. In North America, the U.S. is the largest market for in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market. The US is estimated to hold the largest share in the in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market during the forecast period. The presence of top players and favorable regulations related to product approvals coupled with commercializing new products are the contributing factors for the regional growth. Additionally, the increasing number of R&D activities is the key factor responsible for the Asia-Pacific regional growth for in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance accounting fastest growth of the region during the coming years.
InSilico Trials Technologies, Feops, CADFEM Medical GmbH, Dassault Systèmes, Virtonomy GmbH, Certara Inc., Computational Life, NOVA, TwInsight Medical, Ansys, Inc.; Synopsys, Inc; Sensyne Health plc, Phesi, Tempus, Cerner Corporation are among the leading companies operating in the global in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market.
The pharmaceutical and biopharmaceutical companies segment dominated the global in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market and accounted for the largest market share in 2021.
In-silico trials refers to the development of patient-specific models to form virtual cohorts for testing the safety and/or efficacy of new drugs and medical devices. Also, with the development of computational modelling and simulation entire imaging including source, object, detection, and image interpretation components intended for R&D, optimization, technology assessment, and regulatory evaluation can be achieved.
Rising concerns over animal welfare and benefits and benefits associated with in-silico trials are the most significant factors responsible for the overall market growth.
Based on organization size, large organizations segment took the forefront lead in the worldwide market by accounting largest share in 2021 and is expected to continue to do so till the forecast period.
Trends and growth analysis reports related to Technology, Media and Telecommunications : READ MORE..
The List of Companies - In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market
- InSilico Trials Technologies
- Feops
- CADFEM Medical GmbH
- Dassault Systèmes
- Virtonomy GmbH
- Certara Inc.
- Computational Life
- NOVA
- TwInsight Medical
- Ansys, Inc.
- Synopsys, Inc
- Sensyne Health plc
- Phesi
- Tempus
- Cerner Corporation
 Get Free Sample For
Get Free Sample For