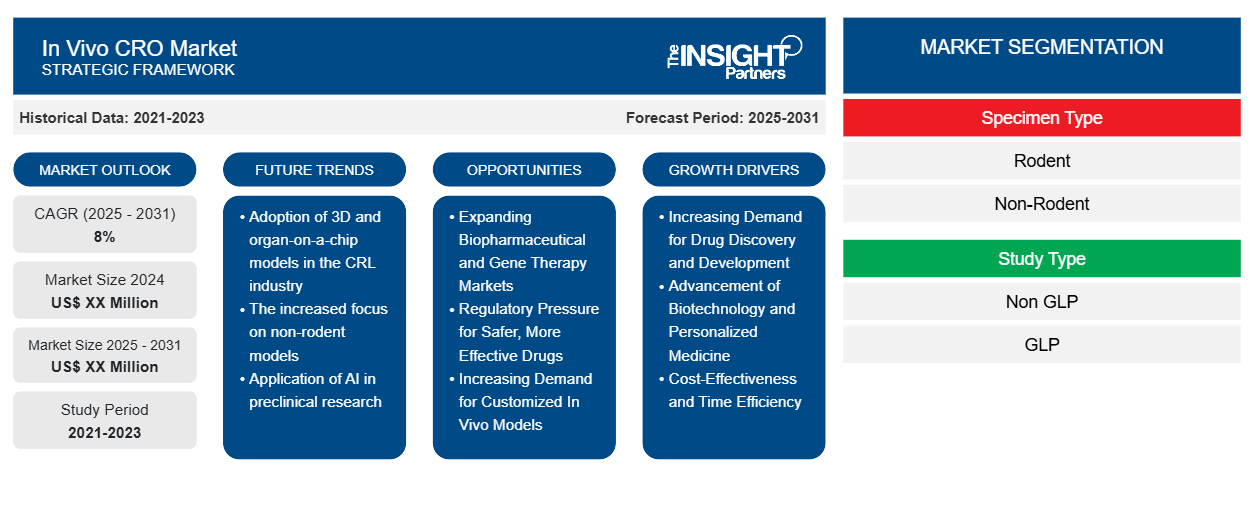
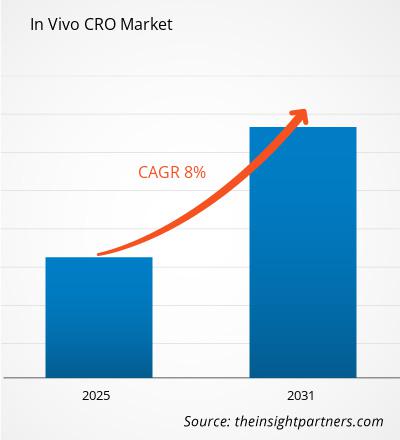
The In Vivo CRO Market is expected to register a CAGR of 8% from 2025 to 2031, with a market size expanding from US$ XX Million in 2024 to US$ XX Million by 2031.
The research report on the in vivo contract research organization (CRO) market is segmented by service type into preclinical services, clinical trial services, and others. Applications analyzed include drug development, toxicology testing, and others. End-users include pharmaceutical companies, biotechnology firms, and academic & research institutions. The regional analysis covers key markets such as North America, Europe, Asia Pacific, the Middle East and Africa, and South America. The market evaluation is presented in US$ for all segmental analyses.
Purpose of the Report
The report In Vivo CRO Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
In Vivo CRO Market Segmentation
Specimen Type
- Rodent
- Non-Rodent
Study Type
- Non GLP
- GLP
Indication
- Autoimmune/Inflammatory Conditions
- Pain Management
- Oncology
- CNS Conditions
- Diabetes
- Obesity
- Other Indications
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
In Vivo CRO Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
In Vivo CRO Market Growth Drivers
- Increasing Demand for Drug Discovery and Development: Benefits from rising new and far more effective therapies, it remains had the allegiance of the in vivo contract research organization (CRO) market. The provision of in vivo CRO services pertaining to animal testing, PK studies, and toxicity assessments have added immense value to preclinical drug discovery and development. There is increasing industry pressure on the global pharmaceutical industry for new drugs to address an increasing tapestry of therapeutic indications including oncology, cardiovascular diseases, and autoimmune disorders. According to PhRMA, there are currently more than 7,000 new drugs in the development pipeline, indicating an increasing demand for preclinical research services. Thereafter, the increased demands stand positioned to set in vivo CROs in demand, hence hastening the whole drug development process and aligning it with regulatory requirements and safety.
- Advancement of Biotechnology and Personalized Medicine: The biotechnology and personalized medicine spheres are also important drivers of request for specialized in vivo CRO services. As the biotechnology industry advances further, it will also create the possibility for in vivo testing for the efficacy and safety of biologics, gene therapies, and cell-based therapies. Personalized medicine itself is also pushing for more refined and well-established preclinical studies to help tailor treatments according to someone's genetic makeup. Because of the intricate and immensely sophisticated therapies available, in vivo CROs come in handy to carry out preclinical studies on such therapies as they see them performing in living organisms; thus, it strongly augurs well for the market's growth.
- Cost-Effectiveness and Time Efficiency: Pharmaceutical and biotechnology companies stand to save big on costs and time by outsourcing in vivo studies to specialized contract research organizations. In particular, the services offered by in vivo CROs very greatly reduce the need for companies to develop their own facilities, personnel, and equipment. This outsourcing model also accelerates the development timelines, as CROs have the resources and experience to conduct studies faster and more efficiently. With the global drug development process becoming increasingly complex and expensive, the ability to reduce operational costs and enhance productivity is a significant driver for the growth of the in vivo CRO market.
In Vivo CRO Market Future Trends
- Adoption of 3D and organ-on-a-chip models in the CRL industry: One of the most significant trends in in vivo CRO markets is the greater adoption of advanced models such as 3D cell cultures and organ-on-a-chip systems. Such innovative approaches facilitate considerably vivid insights into drug interactions, toxicity, and efficacy when compared to conventional analogs. Organ-on-a-chip devices make it possible for organs and tissues to act like human ones so researchers can observe real-time responses to drugs in a controlled environment. This trend paves the way for more predictive and ethical models that replicate human biology well enough to eliminate the need for animal testing in some fields.
- The increased focus on non-rodent models: Standard rodent models (e.g., rats and mice) have commonly been used over the years in in vivo studies; however, there is a significant shift towards using non-rodent models, including non-human primates, rabbits, and pigs. Non-rodent models are frequently preferred because they closely mimic humans in terms of physiology features and are majorly advanced in the studies on drug metabolism and toxicity. The requirement arises from the need for better-quality data for clinical trials and submissions to regulatory bodies. The use of non-rodent models is rising in areas where western medicine depends on significant therapeutic practices like oncology and immunology, aiming to study the possibilities of human responses to drug activities while studying their effects on clinical setups.
- Application of AI in preclinical research: AI is already transforming how in vivo studies are conducted, offering the possibility of linking disparate data sources and creating vast databases of biological information in preclinical research. More specially, it could provide the proper algorithms for dealing with the datasets produced from in vivo experiments. AI is becoming more prevalent to facilitate the analysis of complex datasets this way, provide vital and insight into speedily identifying potential drug candidates, and predict outcomes accurately. The AI-enabled tools can also help optimize study design and reducing animal usage, and enhancing the reproducibility of results. As the integration of AI in research workflows continues to evolve, it is expected to streamline drug development processes, offering more precise insights and accelerating the time to market for new therapies.
In Vivo CRO Market Opportunities
- Expanding Biopharmaceutical and Gene Therapy Markets: The biopharmaceutical sector, particularly the growing market for gene therapies, offers significant opportunities for in vivo CROs. the drug will perform in clinical settings, leading to improved trial success rates. One area of rapid advancement is that of in vivo gene therapy testing. Gene therapies that modify a patient's gene to prevent or treat diseases require complex in vivo work to evaluate the safety and efficacy endpoints. Gene therapy is rapidly becoming a go-to in the treatment of rare and genetic diseases; hence, the need for specialized in vivo test services skyrockets because of it. In vivo CROs with expertise in gene therapy are poised to benefit from the growing biopharmaceutical market as they provide tailored services for gene therapy developers.
- Regulatory Pressure for Safer, More Effective Drugs: With expanding regulatory requirements for the drug approval process, in vivo CROs can help pharmaceutical companies by navigating the increasingly rigid safety and efficacy standards. Regulatory agencies such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) continue to encourage essential preclinical testing—particularly in the areas of safety, immunotoxicity, and drug interactions. In vivo CROs will play a considerable role in assisting companies with these regulatory obstacles before advancing a drug candidate to human clinical trials. Tightening regulatory regimes will unfailingly increase demand for in vivo services that help ensure compliance with global standards.
- Increasing Demand for Customized In Vivo Models: The shift toward personalized medicine and targeted therapeutics is an offer to in vivo CROs for custom testing models. Pharmaceutical companies increasingly seek in vivo models that mirror human conditions and reflect patient-specific features. The CROs providing personalized or patient-derived models, such as patient-derived xenograft (PDX) models, are gaining a competitive advantage. These tailored models allow researchers to make better predictions on how patients will respond to the drugs in clinical settings, subsequently increasing success rates.
In Vivo CRO Market Regional Insights
The regional trends and factors influencing the In Vivo CRO Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses In Vivo CRO Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for In Vivo CRO Market
In Vivo CRO Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ XX Million |
| Market Size by 2031 | US$ XX Million |
| Global CAGR (2025 - 2031) | 8% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Specimen Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
In Vivo CRO Market Players Density: Understanding Its Impact on Business Dynamics
The In Vivo CRO Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the In Vivo CRO Market are:
- American Preclinical Services, LLC.
- Charles River
- Covance Inc.
- GVK Biosciences Private Limited
- Iris Pharma
- MELIOR DISCOVERY
Disclaimer: The companies listed above are not ranked in any particular order.

- Get the In Vivo CRO Market top key players overview
Key Selling Points
- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the In Vivo CRO Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the In Vivo CRO Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
This text is related
to segments covered.

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
The In Vivo CRO Market is expected to register a CAGR of 8% from 2025-2031.
The major driving factors supporting the In Vivo CRO Market growth are- Increasing demand for drug discovery and development, advancement of biotechnology and personalized medicine, and cost-effectiveness and time efficiency are key factors driving the in vivo CRO market.
Key Future Trends in the In Vivo CRO Market are- Adoption of 3D and organ-on-a-chip models in the CRL industry, the increased focus on non-rodent models, and the application of AI in preclinical research are driving advancements in the in vivo CRO market.
Key companies in the In Vivo CRO Market are - American Preclinical Services, LLC., Charles River, Covance Inc., GVK Biosciences Private Limited, Iris Pharma, MELIOR DISCOVERY, Pronexus Analytical AB, Syneos Health, Washington Biotechnology, Inc., WuXi AppTec
The report can be delivered in PDF/PPT format; we can also share excel dataset based on the request.
Some of the customization options available based on request are additional 3–5 company profiles and country-specific analysis of 3–5 countries of your choice. Customizations are to be requested/discussed before making final order confirmation, as our team would review the same and check the feasibility.
Trends and growth analysis reports related to Life Sciences : READ MORE..
1. American Preclinical Services, LLC.
2. Charles River
3. Covance Inc.
4. GVK Biosciences Private Limited
5. Iris Pharma
6. MELIOR DISCOVERY
7. Pronexus Analytical AB
8. Syneos Health
9. Washington Biotechnology, Inc.
10. WuXi AppTec
 Get Free Sample For
Get Free Sample For