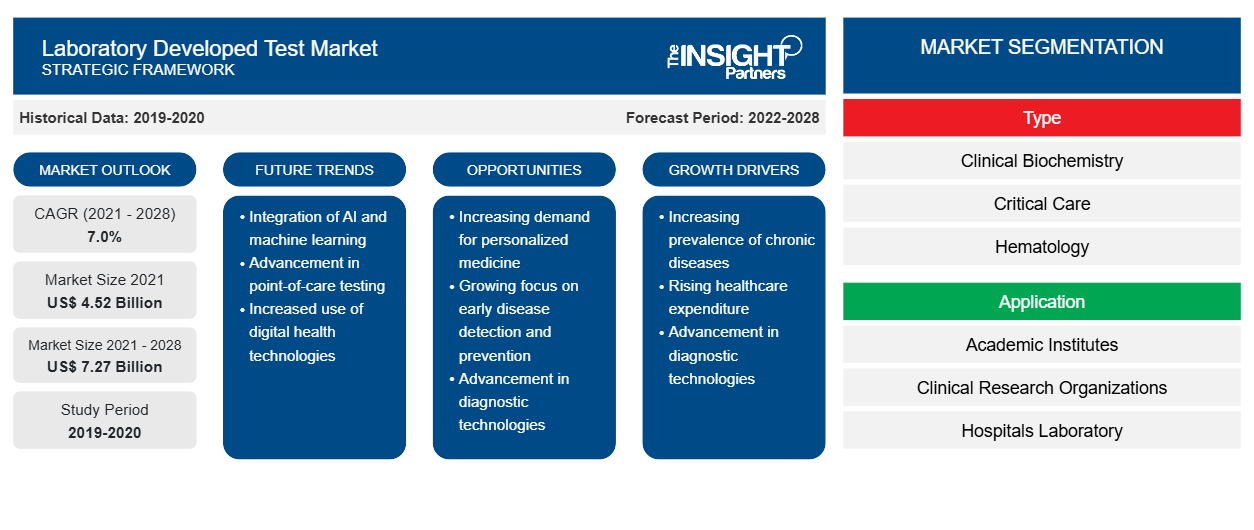
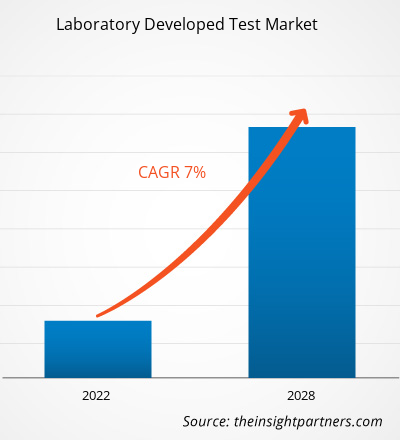
The laboratory developed test market is expected to reach US$ 7,269.3 million by 2028 from US$ 4,524.75 million in 2021; it is expected to grow at a CAGR of 7.0% during 2021–2028.
A laboratory developed test (LDT) is a type of in vitro diagnostic test designed and used within a single laboratory. These tests can be used to estimate or distinguish an analytes such as proteins, biomolecules/compounds (glucose, cholesterol, etc.), and DNA extracted from specimens collected from human subjects. The expansion of automated in vitro diagnostics (IVD) methods for laboratories and dispensaries to render precise and error-free analysis is fueling the laboratory developed test market growth.
The growth of the laboratory developed test market is mainly attributed to factors such as the increasing incidents of cancer and genetic disorders, and large number of product launches. However, changing regulatory landscape is hindering the market growth. For example, in Europe, the In-Vitro Device Regulation (IVDR) compliance will be mandatory for all in vitro diagnostic tests from May 2022; the regulation aims to secure the clinical effectiveness and safety of medical tests, thus transforming the diagnostic industry, which is a great concern for the market players.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Laboratory Developed Test Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Laboratory Developed Test Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Market Insights
Continuous Research on Personalized Medicines Provides Growth Opportunities to Laboratory Developed Test Market Players
LDTs play a vital role in the development of personalized medicines that are likely to prove as promising means of tackling diseases through far eluded effective treatments or cures. As per the Personalized Medicine Coalition, personalized medicines accounted for only 5% of the new FDA-approved molecular entities in 2005; however, in 2016, this number rose to more than 25%. Additionally, 42% of all compounds and 73% of oncology compounds in the pipeline have the potential to serve as personalized medicines. Biopharmaceutical companies have nearly doubled their R&D investments in personalized drugs in the last five years, and they are further expected to increase their investments by 33% in the next five years. Biopharmaceutical researchers also predict a 69% increase in the development of personalized medicines in the next five years. Laboratory tests are used to diagnose illness and predict and monitor drug response as well as to obtain informatics data needed for complex predictive algorithms.
Personalized medicines are becoming the trademark of cancer treatment; it is a constantly evolving approach that is based on the customization of treatments as per the individual genetic makeup. In 2019, the FDA approved 12 personalized medications to investigate and address the root causes of disease, thus combining precision medicine in clinical care. The rising demand for personalized medicine is offering significant growth opportunities for the growth of the players operating in the laboratory developed test market.
Type-Based Insights
The laboratory developed test market, by type, is segmented into clinical biochemistry, critical care, hematology, microbiology, molecular diagnostics, immunology, and others. The molecular diagnostics segment is expected to hold the largest share of the market in 2021. However, the hematology segment is anticipated to register the highest CAGR in the market during the forecast period.
Application-Based Insights
By application, the laboratory developed test market application into academic institutes, clinical research organizations, hospitals laboratory, specialty diagnostic centers, and others. The hospitals laboratory segment is estimated to account for the largest market share in 2021. However, the specialty diagnostic centers segment is anticipated to register the highest CAGR in the market during the forecast period.
Product launches and approvals are commonly adopted strategies by companies to expand their global footprints and product portfolios. Moreover, the laboratory developed test market players focus on the partnership strategy to enlarge their clientele, which, in turn, permits them to maintain their brand name globally.
The report segments laboratory developed test market as follows
Based on type, the laboratory developed test market is segmented into clinical biochemistry, critical care, hematology, microbiology, molecular diagnostics, immunology, and others. Based on Application, the laboratory developed test market is segmented into academic institutes, clinical research organizations, hospitals laboratory, specialty diagnostic centers, and others. On the basis of geography, the laboratory developed test market is segmented into North America (the US, Canada, and Mexico), Europe (the UK, Germany, France, Italy, Spain, and Rest of Europe), Asia Pacific (China, Japan, India, Australia, South Korea, and Rest of Asia Pacific), the Middle East & Africa (the UAE, Saudi Arabia, South Africa, and Rest of the Middle East & Africa), and South and Central America (Brazil, Argentina, and the Rest of South and Central America).
Laboratory Developed Test Market Regional Insights
The regional trends and factors influencing the Laboratory Developed Test Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Laboratory Developed Test Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
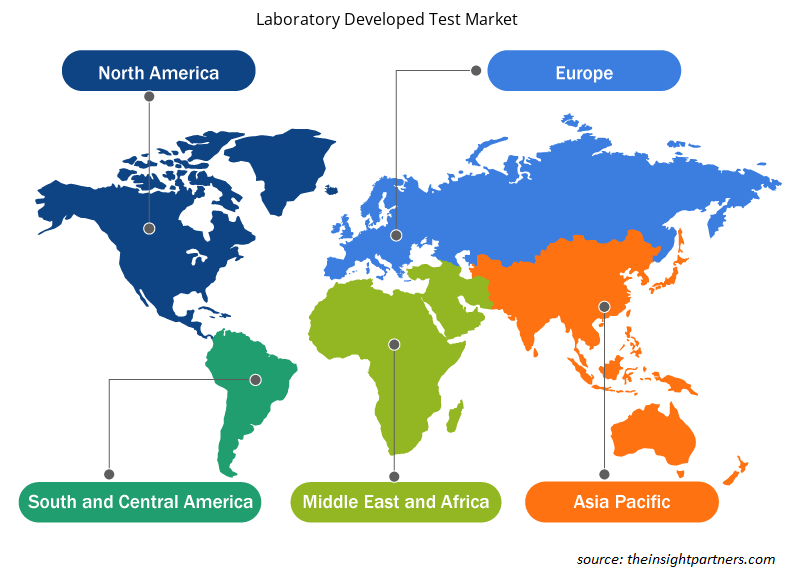
- Get the Regional Specific Data for Laboratory Developed Test Market
Laboratory Developed Test Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 4.52 Billion |
| Market Size by 2028 | US$ 7.27 Billion |
| Global CAGR (2021 - 2028) | 7.0% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
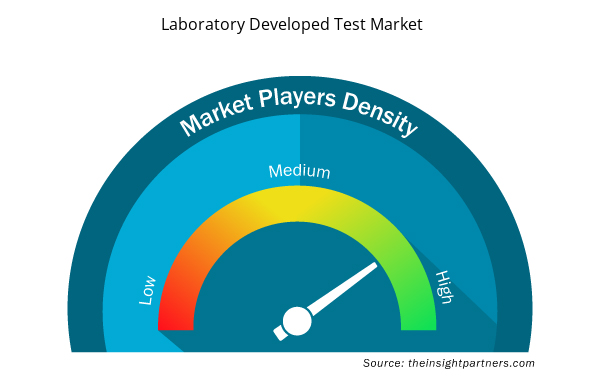
Laboratory Developed Test Market Players Density: Understanding Its Impact on Business Dynamics
The Laboratory Developed Test Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Laboratory Developed Test Market are:
- Quest Diagnostics Incorporated
- F. HOFFMANN-LA ROCHE LTD.,
- QIAGEN
- Illumina, Inc.,
- Eurofins Scientific
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Laboratory Developed Test Market top key players overview
Company Profiles
- Quest Diagnostics Incorporated
- F. HOFFMANN-LA ROCHE LTD.,
- QIAGEN
- Illumina, Inc.,
- Eurofins Scientific
- Biodesix
- Adaptive Biotechnologies
- Biotheranostics
- Rosetta Genomics Ltd.,
- Guardant Health
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Type and Application

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, RoAPAC, RoE, RoMEA, RoSCAM, Saudi Arabia, South Africa, South Korea, Spain, United Arab Emirates, United Kingdom, United States
Frequently Asked Questions
The laboratory developed test market is led by type molecular diagnostics segment is growing significantly due to the increasing attention to the development of genetic therapeutics. The growing research in human genomics is playing a vital role in developing laboratory developed tests. The technological advantages of molecular tests make them compelling diagnostic tools, and they have become valuable for detecting a wide range of genetic diseases. For instance, in November 2017, Edico Genome launched the DRAGEN Clinical Genomics Information System (CGIS).
The automation of LDTs can significantly boost productivity and simplify compliance procedures. Clinical laboratories are under huge pressure to manage increasing test volumes and conduct more complex diagnostic assays, which further underlines the need for flexible automation systems. Many MedTech companies are offering automation solutions to help streamline clinical laboratory processes. For instance, Roche Cobas Omni–Utility Channel is beneficial for both IVD assays and high-volume LDTs. It is designed to meet the growing needs of efficient workflows in laboratories, as it can assist in different stages ranging from sample processing to quick data interpretation. It can run up to 96 results in about 3 hours and up to 864 results in 8 hours, thereby boosting the efficiency of laboratories.
The growth of the region is attributed to factors such as rising public–private partnerships, and increasing funding activities are widely enhancing the performance of medical devices. Moreover, presence of well-developed healthcare infrastructure and government support are some of the prominent factors propelling the market growth in Asia Pacific. In Asia Pacific, India is the largest market for laboratory developed test. The market growth in Asia Pacific is mainly attributed to factors such as the growing usage of laboratory developed test in combating Covid-19, increasing incidents of cancer and genetic disorders, large number of product launches. However concerns high capital investment for setting up advanced labs hinders the market growth in Asia Pacific.
A laboratory developed test (LDT) is a type of in vitro diagnostic test that is designed and used within a single laboratory. These tests can be utilized to estimate or distinguish an extensive assortment of analytes materials such as proteins, chemical compounds like glucose or cholesterol, or DNA, from a specimen received from human anatomy. The expansion of automated in vitro diagnostics (IVD) methods for labs and dispensaries to render precise, and error-free analysis is anticipated to fuel the increment.
The growth of the laboratory developed test market is mainly attributed to factors such as the increasing incidents of cancer and genetic disorders, large number of product launches. However, changing regulatory landscape for instance, in Europe, the In-Vitro Device Regulation (IVDR) compliance will be mandatory for all in vitro diagnostic tests from May 2022; the regulation aims to secure the clinical effectiveness and safety of medical tests, thus transforming the diagnostic industry is a great concern to hinder the market growth. In August 2021, eMed, a telehealth company democratizing healthcare through digital-point-of-care solutions, and Quest Diagnostics the world's leading provider of diagnostic information services, are collaborated to bring clinician-guided rapid antigen testing for COVID-19 to employers seeking to foster safer environments by decreasing the risk of COVID-19 exposure in their workplaces which is contributing to the growth of the target market. Such strategic steps are also projected to drive the market growth.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Laboratory Developed Test Market
- Quest Diagnostics Incorporated
- F. HOFFMANN-LA ROCHE LTD.,
- QIAGEN
- Illumina, Inc.,
- Eurofins Scientific
- Biodesix
- Adaptive Biotechnologies
- Biotheranostics
- Rosetta Genomics Ltd.,
- Guardant Health
 Get Free Sample For
Get Free Sample For