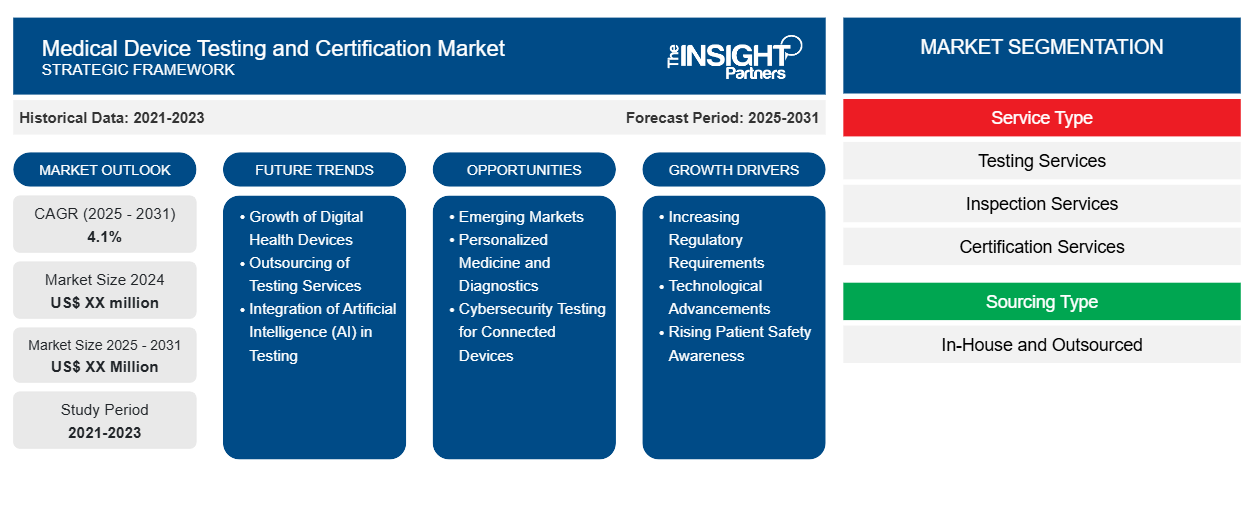
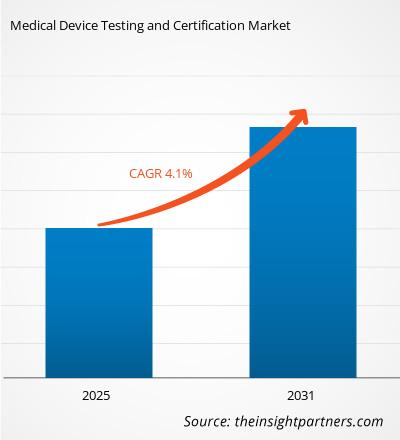
The Medical Device Testing and Certification Market is expected to register a CAGR of 4.1% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The report is segmented by Service Type (Testing Services, Inspection Services, Certification Services, and Other Services), Sourcing Type (In-House and Outsourced), Device Class (Class I, Class II, and Class III), Technology (Active Implant Medical Device, Active Medical Device, Non-Active Medical Device, in Vitro Diagnostic Medical Device, Ophthalmic Medical Device, Orthopedic and Dental Medical Device, and Other Technologies)
Purpose of the Report
The report Medical Device Testing and Certification Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
Medical Device Testing and Certification Market Segmentation
Service Type
- Testing Services
- Inspection Services
- Certification Services
- Other Services
Sourcing Type
- In-House and Outsourced
Device Class
- Class I
- Class II
- Class III
Technology
- Active Implant Medical Device
- Active Medical Device
- Non-Active Medical Device
- in Vitro Diagnostic Medical Device
- Ophthalmic Medical Device
- Orthopedic and Dental Medical Device
- Other Technologies
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Medical Device Testing and Certification Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Medical Device Testing and Certification Market Growth Drivers
- Increasing Regulatory Requirements: As global medical device regulations become stricter, manufacturers must ensure their products comply with various safety and performance standards. This drives demand for testing and certification services, as regulatory bodies require comprehensive evaluations to ensure products meet safety, efficacy, and quality benchmarks, particularly in regions like Europe, the U.S., and Asia.
- Technological Advancements: Rapid advancements in medical technology, such as wearable devices, AI-powered diagnostics, and personalized medicine, require new testing methodologies and certification processes. The increasing complexity of medical devices, including software-driven products, raises the need for specialized testing to ensure functionality, reliability, and user safety, pushing the market for medical device testing and certification.
- Rising Patient Safety Awareness: As healthcare consumers become more informed about safety standards, there is growing pressure on manufacturers to demonstrate the reliability of their devices. Ensuring that medical devices meet rigorous safety standards is critical for avoiding recalls, liabilities, and brand damage, creating a steady demand for testing and certification services to safeguard public health.
Medical Device Testing and Certification Market Future Trends
- Growth of Digital Health Devices:
The digital health market, encompassing telemedicine platforms, mobile health apps, and connected devices, is expanding rapidly. These products require unique testing methods to assess data accuracy, connectivity, and cybersecurity. Certification bodies are evolving to meet the needs of this digital health ecosystem, ensuring that both hardware and software comply with stringent industry standards.
- Outsourcing of Testing Services:
Many medical device manufacturers are increasingly outsourcing testing and certification processes to third-party organizations. This allows companies to focus on innovation and reduce time-to-market, while leveraging specialized expertise for ensuring compliance with complex regulations. This trend is creating opportunities for testing service providers to offer more tailored and efficient solutions to clients.
- Integration of Artificial Intelligence (AI) in Testing:
AI is becoming a key tool in the testing and certification of medical devices. Automated testing and data analytics powered by AI are being used to streamline processes, enhance accuracy, and reduce time in detecting potential issues with medical devices. AI’s role in predictive maintenance and real-time monitoring is also gaining traction in certification processes.
Medical Device Testing and Certification Market Opportunities
- Emerging Markets:
The rising demand for healthcare in emerging economies, such as India, China, and Southeast Asia, presents significant opportunities for the medical device testing and certification market. These regions are increasingly adopting international standards for medical devices, creating a growing need for third-party testing and certification services to facilitate market entry.
- Personalized Medicine and Diagnostics:
As personalized medicine and diagnostic devices gain popularity, there is a growing need for customized testing and certification solutions. Medical devices tailored to specific genetic profiles or treatment regimens require specialized testing to meet evolving safety and performance standards, opening avenues for companies offering innovative testing and certification services.
- Cybersecurity Testing for Connected Devices:
The proliferation of Internet of Things (IoT) devices in healthcare presents a major opportunity for medical device testing companies specializing in cybersecurity. As connected devices become more widespread, ensuring their protection against cyber threats is critical, creating a niche for cybersecurity testing and certification services to ensure data integrity and patient privacy.
Medical Device Testing and Certification Market Regional Insights
The regional trends and factors influencing the Medical Device Testing and Certification Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Medical Device Testing and Certification Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Medical Device Testing and Certification Market
Medical Device Testing and Certification Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ XX million |
| Market Size by 2031 | US$ XX Million |
| Global CAGR (2025 - 2031) | 4.1% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Service Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Medical Device Testing and Certification Market Players Density: Understanding Its Impact on Business Dynamics
The Medical Device Testing and Certification Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Medical Device Testing and Certification Market are:
- TÜV SÜD
- UL LLC
- Intertek Group plc
- Bureau Veritas
- NSF International
- BSI Group
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Medical Device Testing and Certification Market top key players overview
Key Selling Points
- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the Medical Device Testing and Certification Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the Medical Device Testing and Certification Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
This text is related
to segments covered.

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
Increasing regulatory requirements and the need for compliance with safety standards globally are the primary drivers. Manufacturers must ensure their devices meet rigorous regulations to ensure patient safety, avoid recalls, and gain market access
Technological advancements, particularly in digital health and AI-driven devices, are pushing the demand for new testing and certification methods. These innovations require specialized testing protocols to ensure functionality, safety, and data security.
Outsourcing allows manufacturers to save time and focus on core activities such as design and production. By leveraging third-party experts, companies ensure compliance with complex regulations and gain access to specialized testing technologies and knowledge.
Key trends include the rise of digital health devices, outsourcing of testing services, and the integration of AI into testing processes. These trends reflect the need for faster, more accurate testing to meet the demands of modern medical technology.
Opportunities include expanding services in emerging markets, capitalizing on the rise of personalized medicine and diagnostics, and providing cybersecurity testing for connected devices, which are essential for safeguarding patient data in an increasingly interconnected healthcare ecosystem
North America, Europe, and Asia-Pacific are seeing the highest demand. Specifically, regions like the U.S., EU countries, China, and India are expanding their healthcare infrastructure and adopting stricter medical device regulations, fueling demand for certification services.
Trends and growth analysis reports related to Life Sciences : READ MORE..
- T?V S?D
- UL LLC
- Intertek Group plc
- Bureau Veritas
- NSF International
- BSI Group
- SGS SA
- Eurofins Scientific
- DEKRA
- Medical Device Testing Solutions
 Get Free Sample For
Get Free Sample For