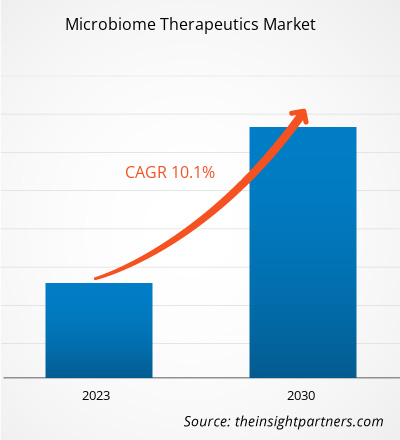
[Research Report] The microbiome therapeutics market size is expected to grow from US$ 375.92 million in 2022 and to reach a value of US$ 813.38 million by 2030; it is anticipated to record a CAGR of 10.1% from 2022 to 2030.
Market Insights and Analyst View:
Microbiome therapeutics, also referred to as microbiome-based therapies or interventions, are medical procedures that use human microbiome's potential to treat a variety of illnesses. In the healthcare and life science industries, microbiome therapeutics are a new and quickly expanding market. This market is being driven by advancements in microbiome research, which are raising awareness of the gut-brain connection and offering the possibility of developing cutting-edge treatments for a range of medical conditions. The pipeline of microbiome therapeutics is dynamic and diverse, aimed at tackling an extensive array of medical conditions and offering novel treatments that harness the human microbiome. More microbiome-based treatments may become available as this field of study develops, opening up new avenues for market expansion. There is a growing need for novel and more efficient treatments due to the rising prevalence of illnesses such as metabolic disorders, obesity, irritable bowel syndrome, and inflammatory bowel disease. Microbiome-based treatments present a cutting-edge method of treating these ailments. This consequently generates a large window of opportunity for microbiome therapeutics market expansion in the near future.
Growth Drivers and Challenges:
Microbiome therapeutics are aimed at modifying the gut microbiome by using bacteriophages, bacteriocins, antibiotics, native or synthetic bacteria, or subtractive or modulatory treatment. By offering individualized, harmonized, dependable, and long-lasting therapy, this strategy can overcome the limitations of traditional therapies. The global therapeutics market has demonstrated its enormous economic potential. Studies have shown promising results in using microbiome therapeutics for conditions such as inflammatory bowel disease, obesity, and even mental health disorders. Ongoing research is also exploring the potential of microbiome-based interventions in areas of cancer treatment, diabetes management, and cardiovascular health. Further, the incidence of gastrointestinal illnesses increases with age, and the GI Alliance data from February 2021 estimates that almost 20 million Americans have chronic digestive diseases, and over 62 million Americans receive a diagnosis of gastrointestinal diseases each year. The International Foundation for Gastrointestinal Disorders, Inc. (IFFGD) released statistics showing that GERD prevalence ranges from 18% to 28% in North America, 23% in South America, and 9–26% in Europe. Therefore, in the upcoming years, the increasing frequency of gastrointestinal disorders will fuel the market expansion.
By understanding the intricate relationship between microbiome and disease development, scientists are developing personalized treatments that target the root cause of the problem rather than merely managing symptoms. As knowledge expands and technologies advance, microbiome therapeutics have the potential to become a cornerstone of modern medicine, offering hope for improved health outcomes for individuals affected by a range of diseases.
Technological advancements have facilitated the development of targeted interventions. By understanding the specific microbial species that are associated with certain diseases or conditions, researchers can develop therapies that specifically target those microbes. This personalized approach has the potential to be more effective and have fewer side effects compared to traditional broad-spectrum antibiotics. In addition to advancements in sequencing technology, other technological innovations have contributed to the development of microbiome therapeutics. Furthermore, there has been progress in the development of synthetic biology tools that allow for the engineering of microbial communities with desired functions. This opens up new possibilities for designing microbiome-based therapies that can be tailored to individual patients.
The process of identifying and isolating specific beneficial microorganisms, as well as producing them in large quantities, can be expensive and time-consuming. Additionally, ensuring the safety and efficacy of these therapeutics requires rigorous testing and regulatory approval, further adding to the cost. The personalized nature of microbiome therapeutics adds another layer of complexity and cost. Each individual's microbiome is unique, meaning that treatments may need to be tailored to their specific microbial composition. This requires extensive analysis and testing that are cost-intensive and time-consuming, thereby hindering the market growth for microbiome therapeutics.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Microbiome Therapeutics Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Microbiome Therapeutics Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Report Segmentation and Scope:
The microbiome therapeutics market is divided on the basis of type, application, and end user. Based on type, the microbiome therapeutics market is segmented into therapeutics and procedures. By application, the microbiome therapeutics market is segmented into metabolic disorders and obesity, C. difficile infection, and inflammatory bowel diseases. In terms of end users, the microbiome therapeutics market is classified as hospitals and clinics, ambulatory surgery centers, and home care. Based on geography, the microbiome therapeutics market is divided into North America (the US, Canada, and Mexico), Europe (the UK, Germany, France, Italy, Spain, and the Rest of Europe), Asia Pacific (China, Japan, India, South Korea, Australia, and the Rest of Asia Pacific), Middle East & Africa (the UAE, Saudi Arabia, South Africa, and the Rest of Middle East & Africa), and South & Central America (Brazil, Argentina, and the Rest of South & Central America).
Segmental Analysis:
Based on type, the microbiome therapeutics market is segmented into therapeutics and procedures. Fecal microbiome therapeutic technique is an emerging technique that has proven to be highly effective in patients with recurring Clostridioides difficile infection (CDI). Growing FMT product approvals by the Food and Drug Administration (FDA) for preventing the recurrence of CDI is fueling the growth of the segment. For instance, in November 2022, the US FDA approved Rebyota, the first fecal microbiota product used for the prevention of CDI recurrence in individuals aged above 18 years.
Based on application, the microbiome therapeutics market is classified into metabolic disorders and obesity, C. difficile infection, inflammatory bowel diseases and others. In 2022, the C. difficile infection segment held the largest share of the market and is expected to register the highest CAGR during 2022–2030. When someone takes antibiotics, good bacteria in the gut are destroyed for a few months. During this time, patients can get sick from Clostridioides difficile, a bacteria that often causes severe or even deadly diarrhea. According to CDC, in 2021, ~118,000 hospitalized patients suffered from C. difficile infection in the US. To prevent and treat C. difficile infection, the gut microbiome of the patient needs to be increased by giving microbiome therapeutics. Due to the increase in the number of C. difficile infections, companies are focusing on developing microbiome therapeutics for the treatment. For instance, in April 2023, the US FDA approved Vowst, the first fecal microbiota used to prevent the recurrence of C. difficile infection.
Based on end user, the microbiome therapeutics market is classified into hospitals and clinics, ambulatory surgery centers, home care, and others. In 2022, the hospitals and clinics segment held the largest share of the market, and it is expected to register the highest CAGR during 2022–2030. Hospitals are the fastest-growing end users of the microbiome therapeutics market. Hospitals are the primary healthcare centers patients seek for their first aid and treatment. The number of patients visiting hospitals is comparatively higher than other healthcare centers. These centers offer various services and provide good-quality treatment to patients. In many hospitals, the treatment fee is reimbursed; also, medicines are readily available in these healthcare centers. The implementation of advanced treatment technologies in hospitals has paved the way for improved and early detection of disease, as well as supported research related to disease treatment. Medical services are increasingly offered due to their rising affordability of treatment and awareness among people. It is a part of the ecosystem of many countries, as it contributes significantly toward the country’s GDP growth.
Microbiome Therapeutics Market Regional Insights
The regional trends and factors influencing the Microbiome Therapeutics Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Microbiome Therapeutics Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Microbiome Therapeutics Market
Microbiome Therapeutics Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 375.92 Million |
| Market Size by 2030 | US$ 813.38 Million |
| Global CAGR (2022 - 2030) | 10.1% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2030 |
| Segments Covered |
By Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Microbiome Therapeutics Market Players Density: Understanding Its Impact on Business Dynamics
The Microbiome Therapeutics Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Microbiome Therapeutics Market are:
- Enterome
- Finch Therapeutics Group Inc
- Caelus Health
- Ferring Pharmaceuticals
- Pendulum Therapeutics Inc.
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Microbiome Therapeutics Market top key players overview
Regional Analysis:
Based on geography, the microbiome therapeutics market is divided into North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America. North America is the most significant contributor to the growth of the microbiome therapeutics market. The increasing understanding of the importance of the human microbiome in maintaining health and preventing diseases has led researchers in the US to explore microbiome-based interventions as a promising approach to treating various conditions. Additionally, the dominance of this country in the microbiome therapeutics market is mainly attributed to its well-developed research infrastructure that favors the development of innovative treatments. Technological advancements in gene sequencing and bioinformatics enable the study and alteration of the microbiome, facilitating the development of personalized microbiome-based interventions. The trend toward personalized medicine aligns well with tailormade microbiome therapeutics. Collaborations between academia, industry, and government organizations are also fueling research and development efforts, accelerating the translation of scientific discoveries into commercial products. Regulatory support and investment in the field further contribute to market growth, as regulatory bodies recognize the potential of microbiome therapeutics and provide guidance for their development and approval. An emphasis on research and innovation has yielded a significant understanding of the impact of gut microbiota on health, providing a robust foundation for microbiome-based therapeutics. A supportive regulatory environment for biotechnology and pharmaceuticals in Canada has encouraged companies to invest in microbiome-focused research and development. Additionally, increased venture capital investments and government funding have infused capital into this research. In January 2020, the Government of Canada, along with its partners, invested US$ 18 million in crucial microbiome research.
Asia Pacific is expected to be the fastest-growing market in the coming years. The microbiome therapeutics market in Japan is experiencing significant growth due to the increasing awareness and research on the human microbiome's impact on health. This has prompted pharmaceutical and biotech companies to invest in microbiome-based therapies. The aging population in Japan and the associated rise in age-related disorders, including gastrointestinal issues and metabolic conditions, create a strong demand for novel treatment options. Microbiome therapeutics, with their potential to provide personalized and effective solutions, are well-suited to address these healthcare challenges. Moreover, a favorable regulatory environment and government initiatives to support microbiome R&D play a crucial role in the microbiome therapeutics market growth in Japan.
Competitive Landscape and Key Companies:
Enterome, Finch Therapeutics Group Inc, Caelus Health, Ferring Pharmaceuticals, Pendulum Therapeutics Inc., AOBiome, Seres Therapeutics, Vedanta Biosciences, COST-BRY Pty Ltd (BiomeBank), and YSOPIA Bioscienceare are a few prominent players operating in the microbiome therapeutics market. These companies focus on expanding service offerings to meet the growing consumer demand worldwide. Their global presence allows them to serve a large set of customers, subsequently allowing them to expand their market share.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Type, Application, End User and Geography

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
The gut microbiota in maintaining human health and inflow of funds to ameliorate microbiome discovery pipeline bolster the microbiome therapeutics market size. However, the high cost of development and production hinders the microbiome therapeutics market growth.
Microbiome therapeutics, also referred to as microbiome-based therapies or interventions, are medical procedures that try to use the human microbiome's potential to treat and promote a variety of illnesses. In the healthcare and life science industries, microbiome therapeutics are a new and quickly expanding market. This market is being driven by advancements in microbiome research, which are raising awareness of the gut-brain connection and offering the possibility of developing cutting-edge treatments for a range of medical conditions.
Fecal microbiome therapeutic technique is an emerging technique that has proven to be highly effective in patients with recurring Clostridioides difficile infection (CDI). Growing FMT product approvals by the Food and Drug Administration (FDA) for preventing the recurrence of CDI is fueling the growth of the segment. For instance, in November 2022, the US FDA approved Rebyota, the first fecal microbiota product used for the prevention of CDI recurrence in individuals aged above 18 years.
The microbiome therapeutics market is expected to be valued at US$ 813.38 million in 2030.
The microbiome therapeutics market was valued at US$ 375.92 million in 2022.
The microbiome therapeutics market has major market players, including Enterome, Finch Therapeutics Group Inc, Caelus Health, Ferring Pharmaceuticals, Pendulum Therapeutics Inc., AOBiome, Seres Therapeutics, Vedanta Biosciences, COST-BRY Pty Ltd (BiomeBank) and YSOPIA Bioscience.
In 2022, the hospitals and clinics segment held the largest share of the market, and it is expected to register the highest CAGR during 2022–2030. Hospitals are the fastest-growing end users of the microbiome therapeutics market. Hospitals are the primary healthcare centers patients seek for their first aid and treatment. The number of patients visiting hospitals is comparatively higher than other healthcare centers. These centers offer various services and provide good-quality treatment to patients. In many hospitals, the treatment fee is reimbursed; also, medicines are readily available in these healthcare centers. The implementation of advanced treatment technologies in hospitals has paved the way for improved and early detection of disease, as well as supported research related to disease treatment..
In 2022, the C. difficile infection segment held the largest share of the market and is expected to register the highest CAGR during 2022–2030. When someone takes antibiotics, good bacteria in the gut are destroyed for a few months. During this time, patients can get sick from Clostridioides difficile, a bacteria that often causes severe or even deadly diarrhea. According to CDC, in 2021, ~118,000 hospitalized patients suffered from C. difficile infection in the US. To prevent and treat C. difficile infection, the gut microbiome of the patient needs to be increased by giving microbiome therapeutics. Due to the increase in the number of C. difficile infections, companies are focusing on developing microbiome therapeutics for the treatment. For instance, in April 2023, the US FDA approved Vowst, the first fecal microbiota used to prevent the recurrence of C. difficile infection.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Microbiome Therapeutics Market
- Enterome
- Finch Therapeutics Group Inc
- Caelus Health
- Ferring Pharmaceuticals
- Pendulum Therapeutics Inc.
- AOBiome
- Seres Therapeutics
- Vedanta Biosciences
- COST-BRY Pty Ltd (BiomeBank)
- YSOPIA Bioscienceare
 Get Free Sample For
Get Free Sample For