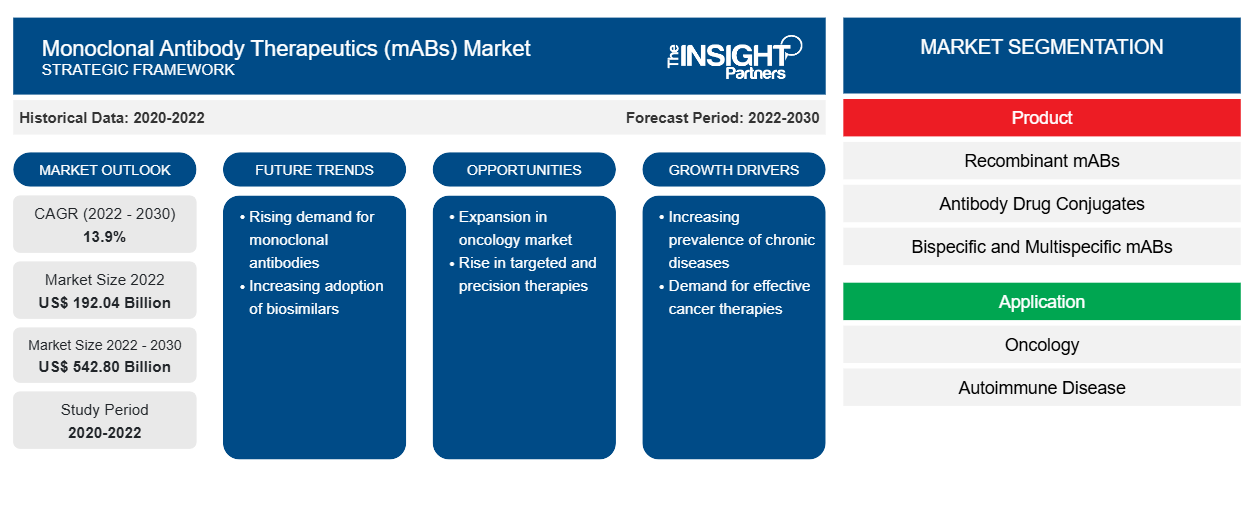
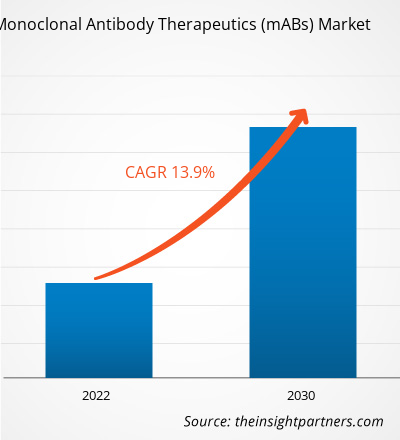
[Research Report] The monoclonal antibody therapeutics (mABs) market size is projected to grow from US$ 192.04 billion in 2022 to US$ 542.80 billion by 2030; the market is estimated to record a CAGR of 13.9% during 2022–2030.
Market Insights and Analyst View:
Robust research and development and the increasing in prevalence of chronic diseases are likely to have a significant impact on the monoclonal antibody therapeutics (mABs) market forecast in the next few years
A monoclonal antibody (mAbs) drug is a homogenous collection of antibodies having specificity toward selected target antigens. The production process of therapeutic mAbs requires a mammalian expression system offering the cell machinery essential to glycosylate, fold, orient, and covalently bind antibody peptide chains to produce complete and biologically functional molecules. New modality antibodies such as bispecific and trispecific antibodies recognize multiple epitopes on a single antigen, while single-domain antibodies can penetrate tissues with greater ease. Such advanced antibody types can enhance the efficiency of the antibody therapeutics, thereby expanding their application areas. These antibodies can also form antibody–drug conjugates to improve the efficiency of chemotherapy agents in targeting specific cell types. Production of mAB-based drugs to treat several diseases propel the market development. Innovative product launches through strategic developments by the manufacturers act as lucrative market opportunities. Furthermore, combination drugs containing mABs acts as a market trend for mAbs therapeutic market.
Market Driver
Production of mAB-Based Drugs to Treat Several Diseases Drives Market Growth
Monoclonal antibody therapeutics (mABs) are employed to treat a wide range of diseases, including cancer, autoimmune diseases, and metabolic diseases. Such drugs produced by biopharmaceutical companies and scientific research institutes have gained significant attention in the global market due to their high specificity, strong targeting ability, and low toxicity and side effects. Thus, an increase in the production capabilities of mAB therapeutics is anticipated to drive the monoclonal antibody therapeutics (mABs) market growth
Therapeutic mABs Approved in European Union (EU) and US
|
|
|
|
|
Pozelimab | VEOPOZ | CHAPLE disease | NA | 2023 |
Elranatamab | Elrexfio | Multiple myeloma | 2023 | 2023 |
Rozanolixizumab | RYSTIGGO | Generalized myasthenia gravis | 2024 | 2023 |
Talquetamab | TALVEY | Multiple myeloma | 2023 | 2023 |
Epcoritamab | EPKINLY | Diffuse large B-cell lymphoma | 2023 | 2023 |
Mirikizumab | Omvoh | Ulcerative colitis | 2023 | 2023 |
Source: Antibody Society
Market Opportunity
Innovative Product Launches Through Strategic Developments by Manufacturers
Organic developments such as product launches by the manufacturers of therapeutic mABs are likely to bolster the monoclonal antibody therapeutics (mABs) market in the coming years. In March 2022, Adagio Therapeutics, Inc. announced the launch of ADG20 (ADINTREVIMAB). The newly launched product is the first monoclonal antibody to meet primary endpoints with statistical significance across pre-and post-exposure prophylaxis and treatment for COVID-19 by seeking US Emergency Use Authorization (EUA).
Further, inorganic developments such as mergers and acquisitions would result in the introduction of new therapeutic mABs. For instance, in July 2023, Elli Lilly announced the acquisition of Versanis, a private clinical-stage biopharmaceutical company intended to treat cardiometabolic diseases. Elli Lilly acquired Versanis to access its core product portfolio, including a monoclonal antibody product named bimagrumab. This product is currently being assessed in the "BELIEVE Phase 2b study" as a standalone molecule. It is also being studied in combination with semaglutide for its combined potential to reduce fat mass, preserve muscle mass, and deliver better patient outcomes in people living with obesity and obesity-related complications. The aforementioned factors are responsible for influential monoclonal antibody therapeutics (mABs) market growth in the coming years.
Monoclonal Antibody Therapeutics (mABs) Market Trends
Combination Drugs Containing Monoclonal Antibodies (mABs)
According to the National Institute of Health (NIH) 2021 report, Roche and Regeneron (pharmaceutical companies) initiated the clinical trial phase 2/3 to evaluate combinational monoclonal antibodies for patients suffering from mild to moderate COVID-19. They are investigating "REGN-COV2," a cocktail drug produced by combining two monoclonal antibodies—casirivimab and imdevimab—for the treatment of COVID-19. These companies expect that the combination of this mAB drug would reduce hospitalization by 70%, and it would be more effective on children above 12 years (having a body weight of more than 40 kg). Researchers are strongly looking for more such therapeutic combinations of monoclonal antibodies. For example, bamlanivimab and etesivimab developed by Elli Lilly have shown positive clinical results for COVID-19 in 2022. Therefore, combination drugs of monoclonal antibodies to treat several diseases would gain significant attention in the coming years, thus emerging as a prominent trend in the monoclonal antibody therapeutics (mABs) market.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Monoclonal Antibody Therapeutics (mABs) Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Monoclonal Antibody Therapeutics (mABs) Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Report Segmentation and Scope:
The monoclonal antibody therapeutics (mABs) market analysis has been carried out by considering the following segments: product, application, and distribution channel
The market, based on product, is segregated as recombinant mABs, antibody–drug conjugates, bispecific and multispecific mABs, biosimilars, and others. The monoclonal antibody therapeutics (mABs) market, by application, is segmented into oncology, autoimmune diseases, and others. The market for autoimmune diseases is further segmented as rheumatoid arthritis, psoriasis, ulcerative colitis, and others. The monoclonal antibody therapeutics (mABs) market, based on distribution channel, is segmented into hospital pharmacies, retail pharmacies, and others.
In terms of product, the recombinant mABs segment held the largest monoclonal antibody therapeutics (mABs) market share in 2022. The antibody–drug conjugates segment is anticipated to record the fastest CAGR of 18.5% during the forecast period. According to the ACS Publications report, therapeutic recombinant monoclonal antibodies reflect state-of-the-art biomedical research conducted by planning effective strategies to treat a wide range of diseases for which no effective treatment is available. Tocilizumab is an example of a recombinant mAB drug administered to treat arthritis, idiopathic arthritis, and rheumatoid arthritis (RA). Additionally, recombinant mABs can also be used to treat diseases such as autoimmune diseases and cancer. Bevacizumab is an example of a recombinant mAB currently used to treat breast, lung, and colorectal cancer; HIV-1; bacterial toxins infections/reactions, and SARS-CoV-2 and ebola virus infections.
Antibody-drug conjugates (ADC) are a rapidly emerging class of therapeutic agents and a new emerging class of highly potent pharmaceutical drugs exploiting a combination of chemotherapy and immunotherapy. According to a report by the NIH, currently, ADCs are predominantly based on immunoglobulin G (IgG), and till now, 13 ADCs have been approved by the US Food and Drug Administration (FDA). Further, more than 90 ADCs are under clinical development/trials.
|
|
|
1 | Mylotarg | Relapsed acute myelogenous leukemia |
2 | Adcetris | Relapsed Hodgkin lymphoma and relapsed systemic anaplastic large cell lymphomas |
3 | Kadcyla | HER2-positive metastatic breast cancer |
4 | Besponsa | Relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia |
5 | Lumoxiti | Relapsed or refractory hairy cell leukemia or HCL |
6 | Polivy | Relapsed or refractory (R/R) diffuse large B-cell lymphoma or DLBCL |
7 | Padcev | Metastatic urothelial cancer |
8 | Enhertu | Metastatic HER2-positive breast cancer |
9 | Trodelvy | Metastatic triple-negative breast cancer |
10 | Blenrep | Relapsed or refractory multiple myeloma |
11 | Zynlota | Large B-cell lymphoma |
12 | Tivdak | Recurrent or metastatic cervical cancer therapy |
13 | Elahere | Platinum-resistant ovarian cancer |
Source: Single Use Support Article
Therefore, regulatory approvals of ADCs and ongoing clinical trials for treatment approaches for rare diseases boost the growth of the monoclonal antibody therapeutics (mABs) market for the antibody–drug conjugates segment during the forecast period.
Regional Analysis:
Based on geography, the Monoclonal Antibody Therapeutics (mABs) market report covers North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America. In 2022, North America accounted for the largest global monoclonal antibody therapeutics (mABs) market share. Asia Pacific is expected to register the highest CAGR during 2022–2030. In North America, the US accounts for the largest market share. Accelerated product approval procedures for mABs therapeutics benefit the market in this country. Until December 2019, 79 therapeutic mABs were approved by the US FDA according to the statistics revealed by a study published in the BioMed Central journal. Among 79 therapeutic mABs, 30 are intended for cancer treatment. In May 2021, the FDA announced authorized EUA for the use of a new therapeutic mAB—Sotrovimab—intended for outpatient applications to treat people suffering from severe COVID-19 condition. In February 2022, the FDA announced the issuing of EUA for bebtelovimab produced by Elli Lilly and Company, an example of mAB intended against the Omicron variant. Further, etesevimab is also an example of therapeutic mABs approved by the US FDA.
Monoclonal Antibody Therapeutics (mABs) Market Regional Insights
The regional trends and factors influencing the Monoclonal Antibody Therapeutics (mABs) Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Monoclonal Antibody Therapeutics (mABs) Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
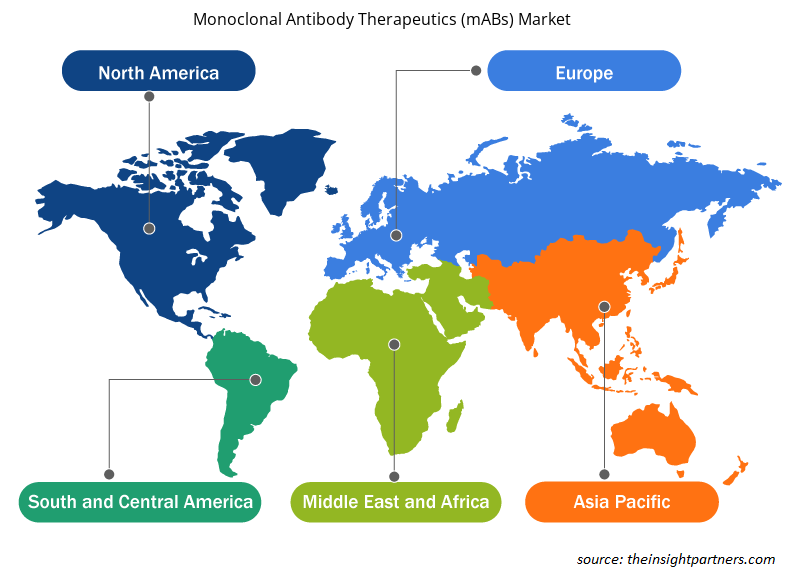
- Get the Regional Specific Data for Monoclonal Antibody Therapeutics (mABs) Market
Monoclonal Antibody Therapeutics (mABs) Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 192.04 Billion |
| Market Size by 2030 | US$ 542.80 Billion |
| Global CAGR (2022 - 2030) | 13.9% |
| Historical Data | 2020-2022 |
| Forecast period | 2022-2030 |
| Segments Covered |
By Product
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Monoclonal Antibody Therapeutics (mABs) Market Players Density: Understanding Its Impact on Business Dynamics
The Monoclonal Antibody Therapeutics (mABs) Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Monoclonal Antibody Therapeutics (mABs) Market are:
- GlaxoSmithKline
- F.Hoffmann-La-Roche
- Bayer AG
- Amgen
- Novartis
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Monoclonal Antibody Therapeutics (mABs) Market top key players overview
Monoclonal antibody therapeutics (mABs) Industry Developments and Future Opportunities:
Various strategic developments by leading players operating in the monoclonal antibody therapeutics (mABs) market are listed below:
- In January 2023, AstraZeneca received approval for Evusheld in the European Union (EU). Evusheld is a combination of two long-acting antibodies—tixagevimab (AZD8895) and cilgavimab (AZD1061). The US government extended support for the development of this product through federal funds from the Department of Health and Human Services, the Administration for Strategic Preparedness and Response, and the Biomedical Advanced Research and Development Authority.
- In August 2023, Regeneron Pharmaceuticals, Inc. entered into an agreement with the Biomedical Advanced Research and Development Authority (BARDA) to support clinical development, clinical manufacturing, and the regulatory licensure process for next-generation monoclonal antibody therapy against COVID-19. Under this agreement, Regeneron plans to work collaboratively with BARDA to evaluate and further develop and manufacture this therapy, and conduct regulatory activities.
Competitive Landscape and Key Companies:
GlaxoSmithKline, F.Hoffmann-La-Roche, Bayer AG, Amgen, Novartis, AbbVie, Bristol-Myers Squibb, Janssen Pharmaceutical, Merck KgaA, and AstraZeneca are among the prominent companies in the monoclonal antibody therapeutics (mABs) market. The monoclonal antibody therapeutics (mABs) market report includes company positioning and concentration to evaluate the performance of key players in the market.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Product, Application, Distribution Channel, and Geography

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
Key factors that are driving the growth of this market are production of mabs-based drugs to treat several infectious diseases is expected to boost the market growth for the monoclonal antibody therapeutics over the years.
The CAGR value of the monoclonal antibody therapeutics market during the forecasted period of 2022-2030 is 13.9%.
The recombinant mABs segment held the largest share of the market in the global monoclonal antibody therapeutics market and held the largest market share in 2022.
Amgen and GlaxoSmithKline are the top two companies that hold huge market shares in the monoclonal antibody therapeutics market.
Monoclonal antibody (mAbs) therapeutics is a homogenous collection of antibodies used to treat illness and are selected to target antigens. Therapeutic mAbs require a mammalian expression system offering the cell machinery required to glycosylate, fold, orient, and covalently bind antibody peptide chains to produce complete and biologically functional molecules. Also, the therapeutic antibody is expanding the use of new modality antibodies such as bispecific and trispecific antibodies to recognize the multiple epitopes on the same antigen and single domain antibodies that can more easily penetrate tissues and antibody-drug conjugates for targeting chemotherapy agents to specific cell types.
The autoimmune disease segment dominated the global monoclonal antibody therapeutics market and held the largest market share in 2022.
The monoclonal antibody therapeutics market majorly consists of the players such GlaxoSmithKline, F.Hoffmann-La-Roche, Bayer AG, Amgen, Novartis, AbbVie, Bristol-Myers Squibb, Janssen Pharmaceutical, Merck KgaA, and AstraZeneca, and amongst others.
Global monoclonal antibody therapeutics market is segmented by region into North America, Europe, Asia Pacific, Middle East & Africa and South & Central America. North America held the largest market share of the monoclonal antibody therapeutics market in 2022.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Monoclonal Antibody Therapeutics (mABs) Market
- GlaxoSmithKline
- F.Hoffmann-La-Roche
- Bayer AG
- Amgen
- Novartis
- AbbVie
- Bristol-Myers Squibb
- Janssen Pharmaceutical
- Merck KgaA
- AstraZeneca
 Get Free Sample For
Get Free Sample For