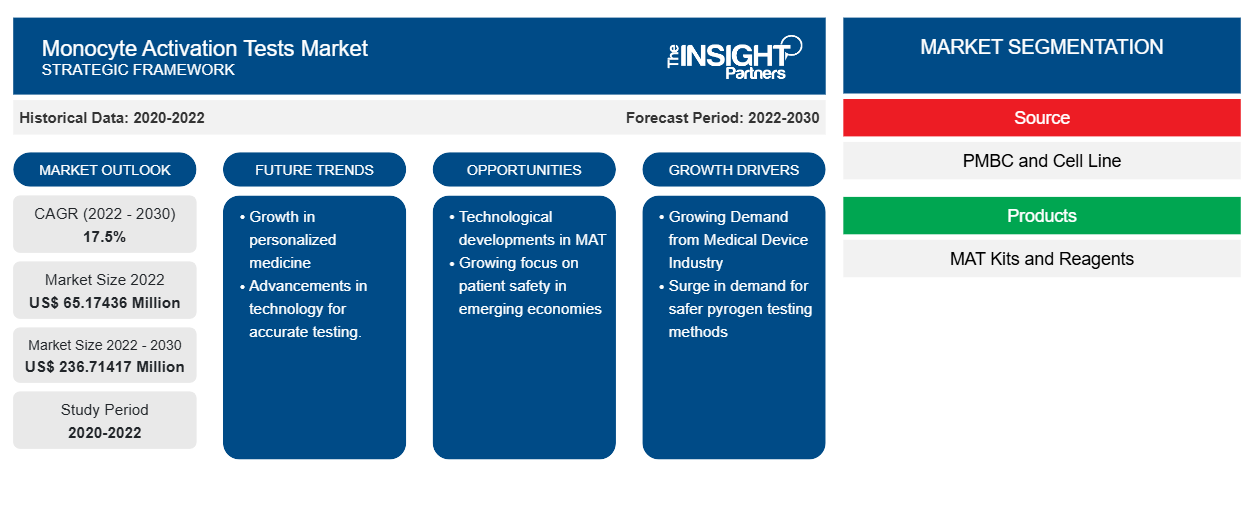
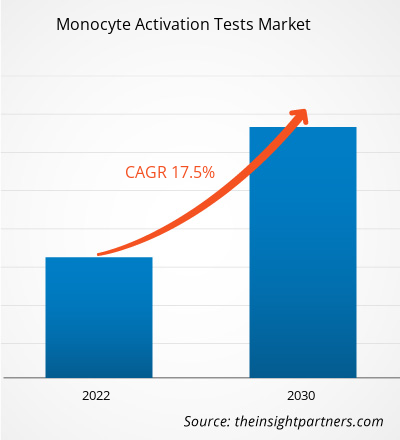
[Research Report] The monocyte activation tests market size was valued at US$ 65,174.36 thousand in 2022 and is expected to reach US$ 236,714.17 thousand by 2030; it is estimated to register a CAGR of 17.5% from 2022 to 2030.
Analyst’s Viewpoint
The monocyte activation tests market analysis includes the study of market drivers such as a rise in safety concerns among patients and a surge in demand for safer pyrogen testing methods in end user industries such as pharmaceutical, biotech, and medical devices. Further, technological developments in monocyte activation test methods are anticipated to propel the monocyte activation tests market growth during the forecast period.
Based on source, the monocyte activation tests market is bifurcated into PMBC and cell line. The PMBC segment held a larger share in 2022 and is expected to continue a similar trend during the forecast period. Based on products, the monocyte activation tests market is bifurcated into MAT kits and reagents. The MAT kits segment held a larger share in 2022 and is expected to continue a similar trend during the forecast period. On the other hand, the reagents segment is anticipated to record a higher CAGR during the forecasted period. Based on application, the monocyte activation tests market is segmented into drug development, vaccine development, medical devices, and others (research etc.). The drug development segment captured the largest share in 2022 and is expected to witness the same trend from 2022 to 2030.
The monocyte activation test detects potentiated cytokine release resulting from the synergistic effect of endotoxin and non-endotoxin pyrogens. The monocyte activation test (MAT) is an in-vitro assay designed to test parenteral drugs, biologics, and medical devices for all classifications of pyrogens. Over the last five years, vaccines that previously used the Rabbit Pyrogen Test as their release assay have been among the initial adopters of the monocyte activation test (MAT). Moreover, unlike MAT, bacterial endotoxin tests are often unsuitable for products that are intrinsically pyrogenic or those that include additives commonly included in vaccines such as aluminum hydroxide, which tend to interfere with the assay. A few instances of MAT's uptake in testing vaccines included Neisseria meningitidis vaccine, Hyperimmune Sera, Meningococcal vaccines, Yellow fever vaccine, Shigella sonnei vaccine, Rabies vaccine, Hepatitis B Vaccine, and Tick-borne encephalitis virus vaccine.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Monocyte Activation Tests Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Monocyte Activation Tests Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Market Insights
Growing Demand from Medical Device Industry
Monocyte activation tests (MATs) are human cell-based tests to detect and quantify pyrogens such as bacteria, fungi, and viruses. MATs use an ELISA assay to measure cytokine release from treated blood cells. MATs are widely available but rarely used in place of animal-based pyrogen tests for biocompatibility assessment of medical devices. The National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM) and the PETA International Science Consortium Ltd. (PISC) convened a September 2018 workshop at the National Institutes of Health to elaborate the necessary steps toward implementation of MAT use in medical device testing. According to Luxoft, a DXC Technology Company, medical devices are aiding the digital transformation of healthcare by providing accurate diagnoses, effective treatments, and personalized care through predictive algorithms and patient data analysis. Technological advancements in personalized medicine, implantable devices, smart medical devices, and noninvasive surgery are revolutionizing the overall healthcare industry by offering better care, improved patient outcomes, and reduced costs. The growth in medical device industry has in-turn enabled the growth of monocyte activation test methods at present and is expected to continue a similar trend during the forecast period.
Market Opportunity
Technological Developments in Monocyte Activation Test Methods
Monocyte activation test methods was mainly introduced as an alternative to animal-based methods and was aimed at offering the opportunity to perform pyrogen testing in a human in vitro system. The Monocyte Activation Test (MAT) was introduced into the European Pharmacopeia (EP) in 2010 following publications of international validations. Continuous innovation and developments in MAT assays and reagents by market participants have essentially led improvements in reproducibility, sensitivity, and specificity, thus making it a reliable and safer option for detecting pyrogens. The MAT assay is used to detect both endotoxins and non-endotoxin pyrogens in parenteral products, such as pharmaceuticals and medical devices. Usually, the MAT gives an in vitro alternative to traditional animal testing in accordance with regulatory guidelines. The Rabbit Pyrogen Test and the Limulus Amebocyte Lysate (LAL) test are widely used for pyrogen detection. Both methods use animals and show a few limitations. The rabbit pyrogen test indicates a lack of robustness as an animal reaction can differ from a human reaction. Furthermore, only endotoxins are detected in the LAL test, resulting in a safety risk by ignoring non-endotoxin pyrogens that might be present in the tested sample. Thus, to overcome these limitations, the monocyte activation test (MAT) was initiated in the European Pharmacopoeia in 2010 as a compendial method to replace the Rabbit Pyrogen Test (EP Chapter 2.6.30) and specified in FDA guidance for industries. Continuous innovation and developments in MAT assays and reagents by market participants have essentially led to improvements in reproducibility, sensitivity, and specificity, thus making it a reliable and safer option for detecting pyrogens.
Report Segmentation and Scope
Source-based Insights
Based on source, the monocyte activation tests market is bifurcated into PMBC and cell line. The PMBC segment held a larger share in 2022 and is expected to continue a similar trend during the forecast period. The same segment is expected to witness a higher CAGR from 2022 to 2030. There are currently two commercialized monocyte activation test cell sources available globally—the Mono-Mac-6 (MM6) cell line and Peripheral Blood Mononuclear Cells (PBMC). The MM6 derives from the blood of a single acute monocytic leukemia patient; as a result, the monocytes sometimes do not have TLRs that reflect the stable expression required to consistently detect pyrogenic contaminants and initiate the release of cytokines by a healthy human. Hence, the reproducibility of MAT results has been found to be low using this cell source. The Ph. Eur. (2.6.30) also describes MM6-based MAT kits as "limited" in their ability to detect non-endotoxin pyrogens. On the other hand, PBMC-based MAT kits source their PBMC from the pooled blood of screened, healthy donors—which means that when incubated with a spiked product sample, the process of monocyte activation can assist the growth of a healthy human being. As a result, results for MAT kits based on this cell source have been consistently found to be reproducible. The Ph. Eur. (2.6.30) counts this cell source as proficient in detecting both endotoxins and non-endotoxin pyrogens.
There are currently three other commercialized PBMC-based monocyte activation test vendors on the market. Each has a LoD of 0.125 EU/ml, 0.02 EU/ml, and 0.016 EU/ml. The CTL-MAT assay has one of the market-leading LoD of 0.004 EU/ml, making it the most sensitive monocyte activation test available worldwide.
Products-based Insights
Based on products, the monocyte activation tests market is bifurcated into MAT kits and reagents. The MAT kits segment held a larger share in 2022 and is expected to continue a similar trend during the forecast period. On the other hand, the reagents segment is expected to register a higher CAGR during the forecasted period.
Application-based Insights
Based on application, the monocyte activation tests market is segmented into drug development, vaccine development, medical devices, and others (research, etc.). The drug development segment captured the largest share in 2022 and is expected to witness the same trend from 2022 to 2030. According to the National Library of Medicine, pharmaceuticals are a group of emergent organic compounds that have contributed to the improving quality of life of patients. The pharmaceutical sector is involved in the production, development, and marketing of branded and generic pharmaceuticals. For the first time in 2014, total pharmaceutical revenues across the globe exceeded US$ 1 trillion. The pharmaceutical market has been expanding at an annual rate of 5.8% since 2017. In the same year, worldwide pharmaceutical market revenue was US$ 1,143 billion and reached US$ 1,462 billion in 2021. The monocyte activation test (MAT) is designed to test parenteral drugs, biologics, and medical devices for all classifications of pyrogens. Parenteral-administered pharmaceutical products must be free of pyrogenic (fever-inducing) contamination as these substances might induce a life-threatening systemic response of the patient’s innate immune system. It is to ensure biological products are free of contaminating pyrogenic material prior to administration to patients. Initially, the RPT and the Bacterial endotoxin Test (BET)/Limulus Amebocyte Lysate Assay (LAL) were used as an ex-vivo option. However, stringent regulations adopted for animal testing methods have forced the market participants to develop an alternative method that minimizes the use of such animal test methods. Considering the limitations of the RPT and the BET and increased manufacturing of complex products, the European Pharmacopoeia introduced MAT activation testing methods that simulate the humane immune response and combine the advantages of the RPT (assessment of pyrogenicity beyond gram-negative endotoxin) with the benefits of an invitro method. In contrast to the RPT, MAT can be applied as a fully quantitative test without the use of animals, making it more appropriate for vaccines that are inherently pyrogenic and are physiologically relevant since they use human cells. The MAT testing assays are able to detect blood-derived products, cell-derived products, and biologics and vaccines. The MAT testing methods can also detect a wide testing range of drug products and medical devices as well as products that are unable to undergo in-vivo testing (for example, products containing hyaluronic acid). Such factors have aided the overall monocyte activation tests market in recent years and are expected to follow a similar trend during the forecast period.
Monocyte Activation Tests Market Regional Insights
Monocyte Activation Tests Market Regional Insights
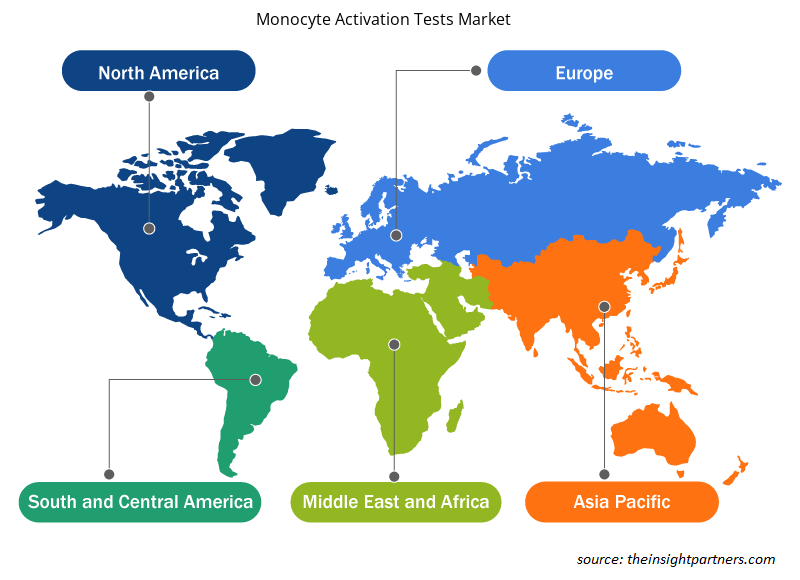
The regional trends and factors influencing the Monocyte Activation Tests Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Monocyte Activation Tests Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Monocyte Activation Tests Market
Monocyte Activation Tests Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 65.17436 Million |
| Market Size by 2030 | US$ 236.71417 Million |
| Global CAGR (2022 - 2030) | 17.5% |
| Historical Data | 2020-2022 |
| Forecast period | 2022-2030 |
| Segments Covered |
By Source
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
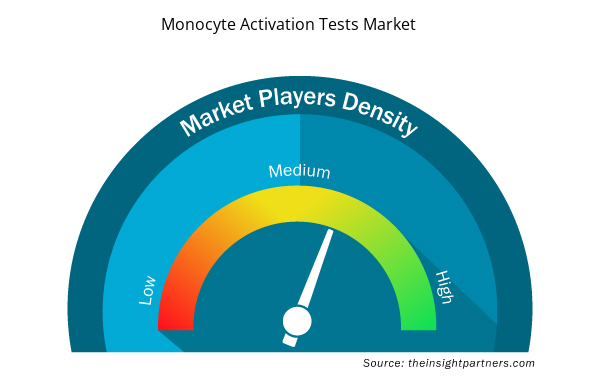
Monocyte Activation Tests Market Players Density: Understanding Its Impact on Business Dynamics
The Monocyte Activation Tests Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Monocyte Activation Tests Market are:
- Merck KGaA, Darmstadt, Germany and/or its affiliates
- Charles River Laboratories International, Inc.
- Thermo Fisher Scientific
- Sanquin
- Lonza Group
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Monocyte Activation Tests Market top key players overview
Regional Analysis
Based on region, the monocyte activation tests market is segmented into North America, Europe, Asia Pacific, South & Central America, and the Middle East & Africa. North America captured the largest market share in 2022 and is expected to continue a similar trend during the forecast period, followed by Europe. Regulatory practices of monocyte activation tests by organizations such as United States Pharmacopeia (USP) and the Government of Canada have further fueled the overall growth of the monocyte activation tests market in the region. Also, increasing focus on patient safety concerns and improved healthcare outcomes is one of the factors aiding the market growth in North America.
Merck KGaA, Darmstadt, Germany and/or its affiliates; Charles River Laboratories International, Inc.; Thermo Fisher Scientific; Sanquin; and Lonza Group are among the leading companies operating in the monocyte activation tests market.
In October 2023, Lonza launched two new rapid monocyte activation test (MAT) systems, the PyroCell MAT Rapid System and PyroCell MAT Human Serum (HS) Rapid System, to streamline and ease rabbit-free pyrogen testing. The systems will replace Lonza’s traditional MAT system kit offerings, and the newly launched products contain the new PeliKine Human IL-6 Rapid ELISA Kit that minimizes hands-on time and reduces time-to-results from two days to two hours. The new tests give pharmaceutical and biotechnology manufacturers easier MAT testing options for product safety testing while helping to reduce the reliance on animals.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Source, Products, and Application

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
Merck KGaA, Darmstadt, Germany and/or its affiliates; Charles River Laboratories International, Inc.; Thermo Fisher Scientific; Sanquin; and Lonza Group are among the leading companies operating in the monocyte activation tests market.
Based on region, the monocyte activation tests market is segmented into North America, Europe, Asia Pacific, South & Central America, and the Middle East & Africa. North America captured the largest market share in 2022 and is expected to continue a similar trend during the forecast period, followed by Europe. Regulatory practices of monocyte activation tests by organizations such as United States Pharmacopeia (USP) and the Government of Canada have further fueled the overall growth of the monocyte activation tests market in the region. Also, increasing focus on patient safety concerns and improved healthcare outcomes is one of the factors aiding the market growth in North America.
Based on source, the monocyte activation tests market is bifurcated into PMBC and cell line. The PMBC segment held a larger share in 2022 and is expected to continue a similar trend during the forecast period. Based on products, the monocyte activation tests market is bifurcated into MAT kits and reagents. The MAT kits segment held a larger share in 2022 and is expected to continue a similar trend during the forecast period. On the other hand, the reagents segment is anticipated to record a higher CAGR during the forecasted period. Based on application, the monocyte activation tests market is segmented into drug development, vaccine development, medical devices, and others (research etc.). The drug development segment captured the largest share in 2022 and is expected to witness the same trend from 2022 to 2030.
The monocyte activation tests market analysis includes the study of market drivers such as a rise in safety concerns among patients and a surge in demand for safer pyrogen testing methods in end user industries such as pharmaceutical, biotech, and medical devices. Further, technological developments in monocyte activation test methods are anticipated to propel the monocyte activation tests market growth during the forecast period.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Monocyte Activation Tests Market
- Merck KGaA, Darmstadt, Germany and/or its affiliates
- Charles River Laboratories International, Inc.
- Thermo Fisher Scientific
- Sanquin
- Lonza Group
- MAT Biotech
- Cellmade Laboratories
- Labor LS SE & Co. KG
- BD Biosciences
- Beckman Coulter
 Get Free Sample For
Get Free Sample For