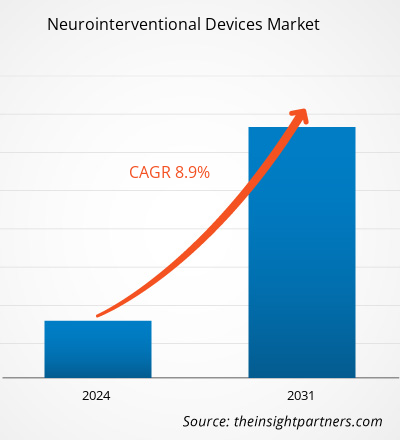
The Neurointerventional Devices Market size was estimated to be US$ 3.00 billion in 2023 and is expected to reach US$ 5.95 billion by 2031; it is estimated to record a CAGR of 8.9% till 2031. Strategic developments and product launches are likely to remain key Neurointerventional Devices Market trends.
Neurointerventional Devices Market Analysis
The key factor that are responsible for the growth of the market include increasing government support, technological advancements, growing geriatric population, increasing preference for minimally invasive surgical procedure, growing awareness about availability of advanced treatment options for neurological devices, and favourable reimbursement policies. In addition, increasing research and development activities in the field of neurological devices is fueling the growth of the neurointerventional devices market.
Neurointerventional Devices Market Overview
Strokes, cerebral aneurysms, and arteriovenous malformation and fistulas are some of the common medical conditions that require neurointerventional devices. Japan is anticipated to register the highest CAGR in the forecast period. The growth of the neurointerventional devices market in Japan is mainly driven by the increasing prevalence of neurological diseases and a rising number of the elderly population in the country. Japan presently has the world's largest elderly population. The country has the fastest aging demographic profile, which is another leading cause of neurovascular diseases. The government has taken multiple measures for treatment and creating awareness about stroke. For instance, the Japan Stroke Association, along with other organizations, organized the draft for the fundamental law on stroke. Moreover, there have been collaborations in the country which enabled the growth of neuroscience industry in Japan.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Neurointerventional Devices Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Neurointerventional Devices Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Neurointerventional Devices Market Drivers and Opportunities
Increasing Prevalence of Neurological Disorders
Neurological diseases are the disorders of the brain, spine, and the nerves that connect them. More than 600 conditions of the nervous system, such as epilepsy, brain tumors, stroke, and Parkinson's disease, are found among the population across the globe. During recent years, the prevalence of neurological disorders has increased significantly. The cases of neurological disorders are increasing globally, which are fueling the growth of the neurointerventional devices. According to Brain Aneurysm Foundation, the annual rate of rupture in brain aneurysm in United States is approximately 8-10 per 100,000 people. As per the same source, about 50,000 deaths worldwide each year caused by brain aneurysms, and half the victims are younger than 50. According to Centers for Disease Control and Prevention (CDC), every year, over 795,000 people in the US have a stroke and over 610,000 of these are first or new strokes. Thus, increase in prevalence neurological diseases is fueling the growth of neurointerventional devices market.
Technological Advancements – An Opportunity of Neurointerventional Devices Market
Continuous technological innovations enhance the efficiency and effectiveness of neurointerventional devices products and services, spanning advancements in materials, manufacturing processes, and digital technologies. Continued investment in R&D to develop innovative neurointerventional devices can help companies differentiate in the market and address unmet clinical needs. This includes development of next-generation devices with improved safety, efficacy, and patient outcomes. In addition, the integration of digital health technologies such as remote monitoring, telemedicine, and data analytics into interventional devices can enhance patient care and improve treatment outcomes. Thus, the growing adoption of technological advanced neurointerventional devices is likely to experience the positive outcomes in the coming future.
Neurointerventional Devices Market Report Segmentation Analysis
Key segments that contributed to the derivation of the Neurointerventional Devices Market analysis are solutions, mode of delivery, application, and end user.
- Based on type, the Neurointerventional Devices Market is segmented into neurovascular thrombectomy devices, neurovascular stents, embolic protection devices, intrasaccular devices, embolic coils, flow diverters, liquid embolic, balloons, and stent retrievers. The neurovascular thrombectomy devices segment held the largest market share in 2023.
- By technique, the Neurointerventional Devices Market is segmented into neurothrombectomy, stenting, coiling procedure, cerebral angiography, and flow disruption. The neurothrombectomy segment held the largest market share in 2023.
- Based on end user, the Neurointerventional Devices Market is segmented into hospitals and ambulatory surgical centers. The hospitals segment held the largest market share in 2023.
Neurointerventional Devices Market Share Analysis by Geography
The geographic scope of the Neurointerventional Devices Market report is mainly divided into five regions: North America, Asia Pacific, Europe, Middle East & Africa, and South & Central America.
North America has dominated the Neurointerventional Devices Market. The market growth in North America is characterized by increasing fraud in healthcare institutions, the presence of key market players, an increase in product launches, increase in number of healthcare institutions, increase in adoption of technologically advanced products, increasing number geriatric population and rising prevalence of neurological disorders. Moreover, Asia Pacific is anticipated to record the highest CAGR in the coming years.
Neurointerventional Devices Market Regional Insights
Neurointerventional Devices Market Regional Insights
The regional trends and factors influencing the Neurointerventional Devices Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Neurointerventional Devices Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Neurointerventional Devices Market
Neurointerventional Devices Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2023 | US$ 3.00 billion |
| Market Size by 2031 | US$ 5.95 billion |
| Global CAGR (2023 - 2031) | 8.9% |
| Historical Data | 2021-2022 |
| Forecast period | 2024-2031 |
| Segments Covered |
By Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Neurointerventional Devices Market Players Density: Understanding Its Impact on Business Dynamics
The Neurointerventional Devices Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Neurointerventional Devices Market are:
- Medtronic
- Boston Scientific Corporation
- Stryker
- iVascular S.L.U.
- Terumo Corporation
- Merit Medical Systems
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Neurointerventional Devices Market top key players overview
Neurointerventional Devices Market News and Recent Developments
The Neurointerventional Devices Market is evaluated by gathering qualitative and quantitative data post primary and secondary research, which includes important corporate publications, association data, and databases. The following is a list of developments in the market for neurointerventional devices and strategies:
- Avicenna.AI received FDA 510(k) clearance for Cina-iPE and Cina-ASPECTS. Cina-iPE enables incidental detection of pulmonary embolism (PE), and Cina-ASPECTS provides automatic assessment of stroke severity. (Source: Bryn Mawr Communications II LLC, Press Release, 2024)
- Cerenovus, Inc., part of Johnson & Johnson MedTech, Inc., launched Cereglide 71 aspiration catheter in Europe. The Cereglide 71 provides direct aspiration of blood clots and for the delivery of compatible stent retrievers—including the company’s Embotrap III revascularization device and the Cerenovus Nimbus geometric clot extractor—into the neurovasculature. (Source: Bryn Mawr Communications II LLC, Press Release, 2024)
Neurointerventional Devices Market Report Coverage and Deliverables
The “Neurointerventional Devices Market Size and Forecast (2021–2031)” report provides a detailed analysis of the market covering below areas:
- Market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Market dynamics such as drivers, restraints, and key opportunities
- Key future trends
- Detailed PEST/Porter’s Five Forces and SWOT analysis
- Global and regional market analysis covering key market trends, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments
- Detailed company profiles
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Type Type ; Technique ; End User and Geography

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
US, Canada, Mexico, UK, Germany, Spain, Italy, France, India, China, Japan, South Korea, Australia, UAE, Saudi Arabia, South Africa, Brazil, Argentina
 Get Free Sample For
Get Free Sample For