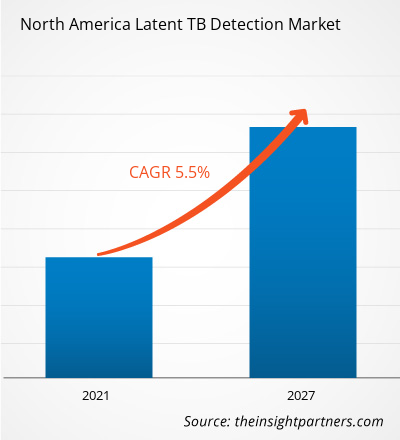
The North America latent TB detection market is expected to grow from US$ 513.29 million in 2020 to US$ 748.25 million by 2027; it is estimated to grow at a CAGR of 5.5% from 2020 to 2027.
The US, Canada, and Mexico are economies in North America. Growing prevalence of tuberculosis is expected to surge the market growth. Tuberculosis (TB) can stay dormant for years before developing into an active TB disease. This condition is known as latent TB. Diagnosis of latent TB is imperative, as it will develop into infectious TB disease when the immune system becomes weak. The World Health Organization (WHO) stated that a total of 1.4 million people died from TB in 2019, making TB one of the top 10 causes of mortality in the region. The data also stated that in 2019 TB affected millions of people in the region, of which 56% were adult males, 32% were adult females, and 12% were children. The disease is prevalent in all age groups in the region. Furthermore, according to the Global Health Education (GHE), a registered charity group, the high TB burden countries accounted for 86% of the estimated cases across the region. The US and Canada are among the countries that are highly affected with TB across the region. Therefore, the high prevalence of TB is driving the need for TB tests, in turn, propelling the growth of the North America latent TD detection market. In case of COVID-19, North America is highly affected specially the US. North America has been witnessing a growing number of COVID-19 cases since its outbreak. As per the CDC, In the US, up to 13 million people may have latent tuberculosis infection. Testing for and treating LTBI is the most effective way to prevent TB infection. Recommended by the CDC, IGRA technology is the required method of TB testing for US Citizenship and Immigration Services (USCIS) immigration exams. The COVID-19 pandemic has threatened the national TB programs worldwide. The WHO have also notified that pandemic is threatening to reverse global progress against tuberculosis. The US is also being affected by the COVID-19 with regards to TB management. CDC’s Division of Tuberculosis Elimination (DTBE) funds 61 state, local, and territorial tuberculosis programs in the US through the TB Elimination and Laboratory cooperative agreement. In April 2020, as part of routine monitoring, CDC communicated with 50 of the 61 (82%) grantees to estimate the effect of COVID-19 on essential TB activities. The observations suggested that the COVID-19 response is diverting resources from essential TB elimination activities. The COVID-19 response has affected multiple sectors of public health, recommended preventive screening, and clinical care in the region. In some TB centers (Mexico), the hospital patient intake system was modified to support COVID-19 admissions, thus severely hindering TB services. In some centers, screening for LTBI was considered a lower priority than screening for COVID-19.
With the new features and technologies, vendors can attract new customers and expand their footprints in emerging markets. This factor is likely to drive the North America latent TB detection market. The North America latent TB detection market is expected to grow at a good CAGR during the forecast period. 
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
North America Latent TB Detection Market Segmentation
North America Latent TB Detection Market – By Test
- Tuberculin Skin Test (TST)
- Interferon Gamma Released Assay (IGRA)
North America Latent TB Detection Market – By End User
- Hospitals
- Diagnostic Centers
- Laboratories
- Others
North America Latent TB Detection Market, by Country
- US
- Canada
- Mexico
North America Latent TB Detection Market -Companies Mentioned
- Abbott
- ARKRAY, Inc.
- BD
- bioMerieux SA
- F. HOFFMANN-LA ROCHE LTD.
- Lionex GmbH
- Oxford Immunotec Ltd
- QIAGEN
- Serum Institute of India Pvt. Ltd.
North America Latent TB Detection Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2020 | US$ 513.29 Million |
| Market Size by 2027 | US$ 748.25 Million |
| Global CAGR (2020 - 2027) | 5.5% |
| Historical Data | 2018-2019 |
| Forecast period | 2021-2027 |
| Segments Covered |
By Test
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Test and Interferon Gamma Released Assay and End User

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
US, Canada
Trends and growth analysis reports related to Life Sciences : READ MORE..
- Abbott
- ARKRAY, Inc.
- BD
- bioMerieux SA
- F. HOFFMANN-LA ROCHE LTD.
- Lionex GmbH
- Oxford Immunotec Ltd
- QIAGEN
- Serum Institute of India Pvt. Ltd.
 Get Free Sample For
Get Free Sample For