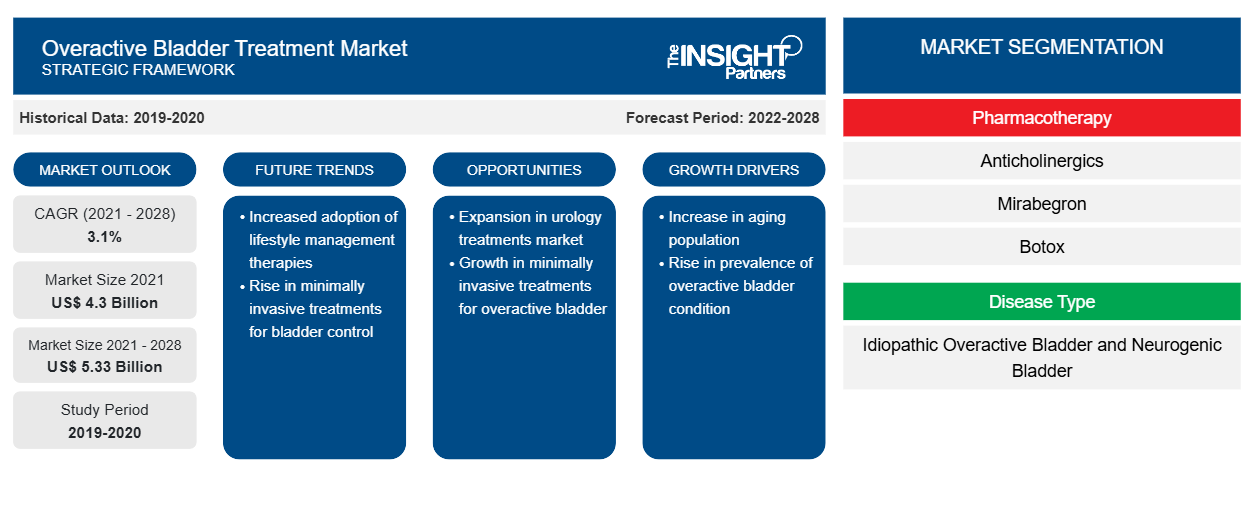
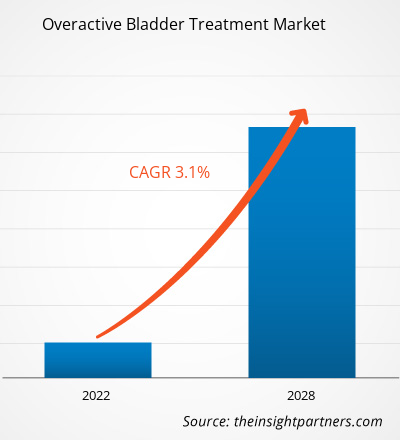
The overactive bladder treatment market is expected to grow from US$ 4,295.93 million in 2021 to US$ 5,333.92 million by 2028; it is estimated to grow at a CAGR of 3.1% from 2022 to 2028.
Overactive bladder (OAB) is the frequent and sudden urge to urinate, which might be difficult to control. OAB can be caused due to abdominal trauma, infection, nerve damage, and certain medications. OAB is common in people aged 65 and older. Women may have OAB after 45 years. The treatment includes changing certain behaviors, medications, and nerve stimulation.
The overactive bladder treatment market is segmented into pharmacotherapy, disease type, and geography. By geography, the market is broadly segmented into North America, Europe, Asia Pacific, the Middle East & Africa, and South & Central America. The overactive bladder treatment market report offers insights and in-depth analysis of the market, emphasizing parameters, such as market trends, market dynamics, and the competitive analysis of the globally leading market players.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Overactive Bladder Treatment Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Overactive Bladder Treatment Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Market Insights
According to the National Association for Incontinence data, in 2018, ~200 million people worldwide were affected by urinary incontinence. Stress incontinence and urge incontinence are the two common types of urinary incontinence affecting women, wherein the latter is also known as overactive bladder. Similarly, a study conducted by a group of researchers at the American College of Physicians and Infectious Diseases Society of America in 2020 stated that urinary tract infection (UTI) was the cause of ~6 million physician visits each year in the US. In Canada, ~500,000 visits to the doctor's office are for UTI consultations annually. UTI is the eighth and fifth most common reason for ambulatory clinic visits and emergency department visits, respectively, in the country. Thus, increasing prevalence of urinary incontinence and growing incidence of urinary tract infections are driving the overactive bladder treatment market.
Following are a few examples of new products launches and approvals:
- In February 2022, Alembic Pharmaceuticals received approval from the US health regulator for its generic version of fesoterodine fumarate extended-release tablets, used for overactive bladder in adults with symptoms of urinary incontinence, urgency, and frequency. The approval by the US Food & Drug Administration (USFDA) for the abbreviated new drug application (ANDA) for fesoterodine fumarate extended-release tablets is for strengths of 4 mg and 8 mg.
- In April 2022, Axonics, Inc. a global medical technology company developing and commercializing novel products for the treatment of bladder and bowel dysfunction—announced the comprehensive launch of the Axonics F15 across the US. The newly developed, long-lived, fully recharge-free sacral neuromodulation (SNM) system received FDA approval in March. The Axonics recharge-free system is a welcome advancement for patients suffering from bladder and bowel dysfunction.
- In March 2021, Astellas Pharma's Myrbetriq—used for the treatment of neurogenic detrusor overactivity (NDO) in pediatric patients aged three years and older (weighing 35 kg or more)—and Myrbetriq Granules—for the treatment of NDO in pediatric patients aged three years and older—were approved by the US Food and Drug Administration (FDA).
Such product developments by market players are driving the overactive bladder treatment market.
Type Insights
Based on pharmacotherapy, the overactive bladder treatment market is segmented into mirabegron, botox, neurostimulation, anticholinergics, and intravesical instillation. The mirabegron segment held the largest share of the market in 2021 and is anticipated to register the highest CAGR during the forecast period. Mirabegron is used alone or with solifenacin to treat overactive bladder in adults. It is used to treat neurogenic detrusor overactivity (a bladder control condition caused by the brain, spinal cord, or nerve problem) in children aged three years or more. Mirabegron belongs to the class of drugs called beta-3 adrenergic agonists. It relaxes the bladder muscles to prevent urgent, frequent, or uncontrolled urination. Mirabegron is available as an extended-release (long-acting) tablet and an extended-release suspension that can be taken orally. In October 2022, Zydus Pharmaceuticals Inc. received approval from the USFDA to market Mirabegron Extended-Release Tablets USP 25 mg and 50 mg. The increasing demand for this therapy is expected to fuel the growth of the overactive bladder treatment market during the forecast period.
Services Insights
Based on pharmacotherapy, the overactive bladder treatment market is segmented into mirabegron, Botox, neurostimulation, anticholinergics, and intravesical instillation. The neurostimulation segment is anticipated to register the highest CAGR in the market during the forecast period. Neurological control of the bladder is complex and depends on different spinal, central, and peripheral nerves, and their multiple reflex pathways. Neurological control is performed at different human body sites and, therefore, targets various nerves. Some of these target nerves are directly involved in lower urinary tract sensory-motor control, such as sacral or pudendal nerves, while others are more indirectly involved. Electrical stimulation is used to activate these nerves to control the bladder.
Partnerships and collaborations are highly adopted strategies by the global overactive bladder treatment market players. A few of the recent key market developments are listed below:
- In April 2021, Medtronic plc announced approval from the US Food and Drug Administration (FDA) to proceed with an investigational device exemption (IDE) trial to evaluate its internally developed implantable tibial neuromodulation (TNM) device a therapy designed to provide relief from symptoms of bladder incontinence.
- In July 2020, Hisamitsu Pharmaceutical Co., Inc. announced the approval of manufacturing and marketing for OABLOK PATCH in Thailand. The product is a systemic transdermal formulation developed using Hisamitsu’s Transdermal Drug Delivery System (TDDS) technology for treating overactive bladder with symptoms such as urinary urgency and frequent urination.
- In July 2019, Teva Pharmaceuticals Ltd. launched Solifenacin Succinate Tablets, which are muscarinic antagonists indicated for the treatment of overactive bladder with symptoms of urinary incontinence and increase in urinary frequency.
The COVID-19 pandemic has shown some favorable scenarios for players operating in the overactive bladder treatment market. The US was the highly affected country in North America. The infection severely affected the geriatric population in the country, causing various complications and leading to the death of a large population. For instance, as of May 16, 2022, the US reported 84,230,829 COVID cases with 1,026,670 deaths. Therefore, overactive bladder treatment and proper care are required to prevent chronic cases of infection and health conditions.
After the COVID-19 outbreak, there has been an increase in the occurrence of OAB. According to a study by American Urological Association, patients with COVID-19 infections were at an increased risk for developing new or worsening overactive bladder symptoms. Moreover, approximately one-third of patients with COVID-19 reported a significant increase in clinical symptoms during the International Consultation on Incontinence Questionnaire Overactive Bladder Module (ICIQ-OAB) conducted two months after infection. Out of these one-third of patients, approximately 1 in 5 patients, reported that their OAB symptoms were new. This increased prevalence of OAB due to COVID-19 positively impacted the demand for overactive bladder treatment during the pandemic.
Overactive Bladder Treatment Market Regional Insights
Overactive Bladder Treatment Market Regional Insights
The regional trends and factors influencing the Overactive Bladder Treatment Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Overactive Bladder Treatment Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Overactive Bladder Treatment Market
Overactive Bladder Treatment Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 4.3 Billion |
| Market Size by 2028 | US$ 5.33 Billion |
| Global CAGR (2021 - 2028) | 3.1% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Pharmacotherapy
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Overactive Bladder Treatment Market Players Density: Understanding Its Impact on Business Dynamics
The Overactive Bladder Treatment Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Overactive Bladder Treatment Market are:
- Alembic Pharmaceuticals Limited
- Astellas Pharma Inc
- Pfizer Inc
- AbbVie Inc
- Teva Pharmaceutical Industries Ltd
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Overactive Bladder Treatment Market top key players overview
Overactive Bladder Treatment – Market Segmentation
The global overactive bladder treatment market is analyzed based on pharmacotherapy, disease type, and geography. Based on pharmacotherapy, the overactive bladder treatment market is classified into mirabegron, Botox, neurostimulation, anticholinergics, and intravesical instillation. Based on disease type, the market is divided into idiopathic overactive bladder and neurogenic bladder.Geographically, the overactive bladder treatment market is segmented into North America, Europe, Asia Pacific, the Middle East & Africa, and South & Central America.
Company Profiles
- Alembic Pharmaceuticals Limited
- Astellas Pharma Inc
- AbbVie Inc
- Teva Pharmaceuticals Industries Ltd
- Endo Pharmaceuticals Inc
- Hisamitsu Pharmaceutical Co. Inc
- Medtronic Plc
- Colorado Urology Associates, PLLC
- Axonics Modulation Technologies, Inc
- Pfizer Inc
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Pharmacotherapy, and Disease Type

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, Saudi Arabia, South Africa, South Korea, Spain, UAE, UK, US
Frequently Asked Questions
The factors that are driving the growth of the overactive bladder treatment market are urinary tract infections (UTI) which lead to increased activity in the bladder wall muscles, causing symptoms like an overactive bladder. The bacteria causing UTIs reside in the lining of urothelial cells, and they attack the host body when their innate immunity is low. Individuals with recurrent UTIs are found to have voiding dysfunction and detrusor overactivity, and these functional abnormalities may further damage the integrity of the urothelial barrier. The increasing prevalence of UTIs, coupled with the association of UTIs with overactive bladder, is one of the prominent factors contributing to the overactive bladder treatment market growth.
The CAGR value of the overactive bladder treatment market during the forecasted period of 2022-2028 is 3.1%.
The overactive bladder treatment market is estimated to be valued at US$ 4,295.93 million in 2021.
The overactive bladder treatment market is expected to be valued at US$ 5,333.92 million in 2028.
The overactive bladder treatment market majorly consists of the players, such as Alembic Pharmaceuticals Limited, Astellas Pharma Inc; Pfizer Inc; AbbVie Inc; Teva Pharmaceutical Industries Ltd; Endo Pharmaceuticals Inc; Hisamitsu Pharmaceutical Co., Inc; Medtronic Plc; Colorado Urology Associates, PLLC; Axonics Modulation Technologies, Inc.
The mirabegron segment held the largest share of the market in 2021. However, neurostimulation is expected to grow at the highest CAGR during the forecast period.
The Asia Pacific is expected to be the fastest-growing region in the overactive bladder treatment market over the forecast period due to the rising geriatric population, increasing urinary incontinence cases, and a surge in product launches.
The sudden need to urinate causes a problem with bladder dysfunction. An overactive bladder may affect how often you pee and your urgency. Causes include abdominal trauma, infection, nerve damage, medications, and certain fluids. Treatment includes changing certain behaviors, medications, and nerve stimulation. Overactive bladder is common in people aged 65 and older. Women may have OAB at a younger age, usually around 45.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies -Overactive Bladder Treatment Market
- Alembic Pharmaceuticals Limited
- Astellas Pharma Inc
- Pfizer Inc
- AbbVie Inc
- Teva Pharmaceutical Industries Ltd
- Endo Pharmaceuticals Inc.
- Hisamitsu Pharmaceutical Co.,Inc
- Medtronic Plc
- Colorado Urology Associates, PLLC
- Axonics Modulation Technologies, Inc
 Get Free Sample For
Get Free Sample For