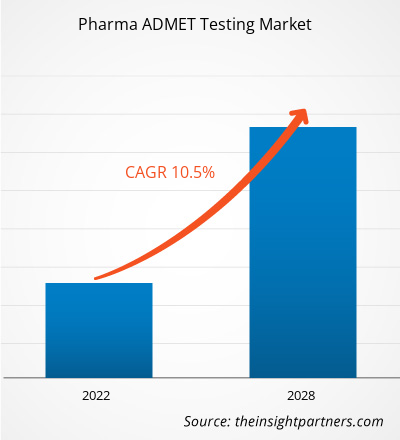
The pharma ADMET testing market is expected to grow from US$ 6,783.06 million in 2021 to US$ 13,578.62 million by 2028; it is estimated to grow at a CAGR of 10.5% from 2022 to 2028.
The rising number of product approvals and launches drives the market growth. The pharma ADMET testing market comprises major competitive players who adopt various strategies, including product launches, geographic expansion, and technological advancements. A few recent developments in the market are listed below:
- In December 2021, Discovery Life Sciences announced the acquisition of In Vitro ADMET Laboratories (IVAL) to leverage the capabilities of the world's largest hepatocyte inventory and a world-renowned scientific leader. IVAL, Maryland, offers physiologically accurate in vitro experimental systems to increase the effectiveness of toxicology, pharmacology, and drug metabolism testing for the pharmaceutical industry.
- In April 2022, Discovery Life Sciences, a company employing biospecimen and biomarker experts, announced the purchase of the Gentest business unit of Corning Incorporated's Life Sciences division. Gentest will be added to the recently acquired In Vitro ADMET Laboratories, LLC (IVAL) by Discovery Life Sciences. This will make Discovery a prominent provider of in vitro drug trial systems to the pharmaceutical and life sciences industries.
- In October 2021, Genetic Analysis AS (GA), a company specializing in molecular diagnostics, announced a service agreement with Eurofins ADME BIOANALYSES, a Contract Research Organization (CRO) that offers the pharmaceutical industry a range of services in the areas of pharmacokinetics, pharmacodynamics, and drug metabolism.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Pharma ADMET Testing Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Pharma ADMET Testing Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
The active participation of market players in product innovation and development, coupled with an increase in approvals of products, fuels the growth of the pharma ADMET testing market. Also, with the advent of advanced technologies, the market would grow exponentially during the forecast period.
The high cost of ADMET testing studies hinders the growth of the pharma ADMET testing market. As per the Research and Development in the Pharmaceutical Industry, a 2021 report by Congressional Budget Office, the projected cost to develop a new drug can range from less than US$ 1 billion to more than US$ 2 billion, including capital expenses and treatment costs (for patients administered with drugs that don't make it to market). The pharmaceutical industry dedicated US$ 83 billion to R&D expenditures in 2019. These expenditures entailed costs incurred by discovering and testing new drugs, developing incremental innovations such as product extensions, and clinical testing for safety monitoring or marketing purposes. This amount is ~10-times what the industry spent per year in the 1980s. As a result of the unpredictability and high cost of discovering and developing new drugs, only ~12% of medications that undergo clinical trials ultimately receive FDA approval for market launch. Such factors are hindering the growth of the global pharma ADMET testing market.
Regional Overview
Asia Pacific registers the highest CAGR of the pharma ADMET testing market. High demand for toxicology testing products and precision medicines, increasing discovery of drugs, and rising investments in R&D by key market players are among the factors bolstering the growth of the Asia Pacific market.The pharma ADMET testing market in China is expanding due to the country's increasing population suffering from chronic diseases leading to the demand for personalized medicine. Drug discovery in China is booming owing to the support from institutes and organizations promoting in-vitro ADMET testing of the drugs. For instance, Institute for In-Vitro Sciences (IIVS) has worked collaboratively with international governments to help them implement non-animal test methods for the regulation of products and ingredients. In April 2019, IIVS recognized China’s National Medical Products Administration (NMPA) for their acceptance of certain non-animal (alternative) test methods for the regulation of cosmetics.
Moreover, key players operating in the pharma ADMET testing market in China are following organic and inorganic strategies to expand their portfolio and presence across APAC. For instance, in June 2022, WuXi ATU and Wugen Inc. announced a partnership to produce Wugen’s WU-NK-101, a novel immunotherapy that harnesses the power of memory natural killer (NK) cells to treat cancers. Under the partnership, WuXi ATU agreed to provide manufacturing and testing services for WU-NK-101 to enable the delivery of the innovative cell therapy product to cancer patients.
The pharma ADMET testing market in South Korea is growing gradually due to the increased emphasis on drug safety data, new demand for health economics and outcomes research, and shift toward personalized medicine and orphan drug development. South Korea is found to be the most attractive early phase destination based on the availability of global and regional suppliers, patient pool, and the current regulatory scenario of the country. The interest and growth in South Korea in Phase I-IIa studies relative to other areas in Asia, and even relative to the rest of the world, is directly related to the following factors:
- Investment by the South Korean government in developing their biotechnology industry
- Highly educated medical and scientific staff interested in early clinical research
- Access to patients and focus on quality
- Ever-expanding IT infrastructure.
The above factors are propelling the expansion of several CROs across South Korea. Additionally, South Korea announced the launch of a 5-year plan for advancing clinical trials and boosting the country's status as a research destination across the world. In 2019, Novotech company announced a partnership with two major hospitals in South Korea responsible for strengthening Novotech's clinical service capabilities in the country. These factors are expected to fuel the growth of the pharma ADMET testing market in Asia Pacific during the forecast period.
Application Insights
Based on application, the global pharma ADMET testing market is segmented into systemic toxicity, renal toxicity, hepatotoxicity, neurotoxicity, and others. The systemic toxicity segment is estimated to account for the largest market share during the forecast period. Pharma ADMET testing is performed to identify or spot the toxic effects of drug molecules on organs in the body. If the drug molecule is toxic then it may cause systemic toxicity or organ toxicity. A systemic toxicity affects the entire body or many organs rather than a specific site. ADMET testing finds its application in performing different chemical, pharmacological, and genetic tests that support overall drug discovery activities, including drug trials, drug designs, and other drug interactions. Moreover, there are several drug categories and drug molecules that cause systemic toxicity. Local anesthetic systemic toxicity (LAST) is a life-threatening adverse event that may occur after the administration of local anesthetic drugs through a variety of routes. The increasing use of local anesthetic techniques in various healthcare settings makes contemporary understanding of LAST highly relevant. Additionally, pharma ADMET testing plays a critical role for identifying molecular toxicity and resolving it at the preclinical drug development phase. A study published in Springer Nature in February 2022 entitled “In Silico Models for Predicting Acute Systemic Toxicity” reveals the use of structure-based computational models available and potentially useful in the assessment of acute systemic toxicity.
The hepatotoxicity segment is expected to register the highest CAGR during the forecast period. Hepatotoxicity is the injury or damage to the liver caused by drugs. It is a known and serious adverse drug reaction. Moreover, drug-induced hepatotoxicity is a leading cause of attrition during drug development. In vitro three-dimensional (3D) cell cultures allow better recapitulation of the complex in vivo microenvironment than traditional 2D monolayer models. Thus, the use of such an advanced model to study hepatotoxicity is expected to propel the pharma ADMET testing market growth for this segment during 2021–2028.
Companies operating in the pharma ADMET testing market adopt the product innovation strategy to meet the evolving customer demands across the world, which also permits them to maintain their brand name in the market.
Pharma ADMET Testing Market Regional Insights
Pharma ADMET Testing Market Regional Insights
The regional trends and factors influencing the Pharma ADMET Testing Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Pharma ADMET Testing Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Pharma ADMET Testing Market
Pharma ADMET Testing Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 6.78 Billion |
| Market Size by 2028 | US$ 13.58 Billion |
| Global CAGR (2021 - 2028) | 10.5% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Testing Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Pharma ADMET Testing Market Players Density: Understanding Its Impact on Business Dynamics
The Pharma ADMET Testing Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Pharma ADMET Testing Market are:
- CMIC HOLDINGS Co., LTD
- Charles River Laboratories
- Wuxi AppTec
- Promega Corporation
- MERCK KGaA
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Pharma ADMET Testing Market top key players overview
Global Pharma ADMET Testing Market – Segmentation
The pharma ADMET testing market is segmented on the basis of testing type, technology, application, and geography. Based on testing type, the market is segmented into in vivo ADMET testing, in vitro ADMET testing, and in silico ADMET testing. Based on technology, the market is segmented into cell culture, high throughput, molecular imaging, and OMICS technology. Based on application, the global pharma ADMET testing market is segmented into systemic toxicity, renal toxicity, hepatotoxicity, neurotoxicity, and others. By geography, the market is segmented into North America (the US, Canada, and Mexico), Europe (the UK, Germany, France, Italy, Spain, and the Rest of Europe), Asia Pacific (China, Japan, India, Australia, South Korea, and the Rest of Asia Pacific), the Middle East & Africa (the UAE, Saudi Arabia, South Africa, and the Rest of the Middle East & Africa), and South & Central America (Brazil, Argentina, and the Rest of South & Central America).
Company Profiles
- CMIC Holdings Co., Ltd.
- Charles River Laboratories
- WuXi AppTec
- Promega Corporation
- Merck KGaA
- Agilient Technologies, Inc.
- Biovia (Dassault Systemes)
- Cyprotex Limited
- Bio-Rad Laboratories
- IQVIA Inc.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Testing Type, Technology, and Application

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, Saudi Arabia, South Africa, South Korea, Spain, United Arab Emirates, United Kingdom, United States
Frequently Asked Questions
CMIC HOLDINGS Co., LTD; Charles River Laboratories; WuXi AppTec; Promega Corporation; MERCK KGaA; Agilent Technologies, Inc.; Biovia (Dassault Systèmes); Cyprotex Limited; Bio-Rad Laboratories, Inc.; and IQVIA Inc. are among the leading companies operating in the global pharma ADMET testing market.
Based on testing type, the market is segmented into in vivo ADMET testing, in vitro ADMET testing, and in silico ADMET testing. In vivo ADMET testing segment is anticipated to hold a larger share in 2022 and is expected to continue to do so during the forecast period.
High rate of late-stage drug failure and the increasing number of product approvals and launches are the most significant factors responsible for the overall market growth.
ADMET testing expands as for absorption, distribution, metabolism, elimination, and toxicology testing of the drug molecule. These drug tests define the impact of a specific drug or chemical on human tissues. Toxicology testing is an important phenomenon before introducing new medicine to the market. ADMET testing facilitates pharmaceutical manufacturing companies to minimize their drug discovery time, and testing complications, and reduce the cost of drug development.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Pharma ADMET Testing Market
- CMIC HOLDINGS Co., LTD
- Charles River Laboratories
- Wuxi AppTec
- Promega Corporation
- MERCK KGaA
- Agilent Technologies, Inc.
- Biovia (Dassault Systèmes)
- Cyprotex Limited
- Bio-Rad Laboratories, Inc.
- IQVIA Inc.
 Get Free Sample For
Get Free Sample For