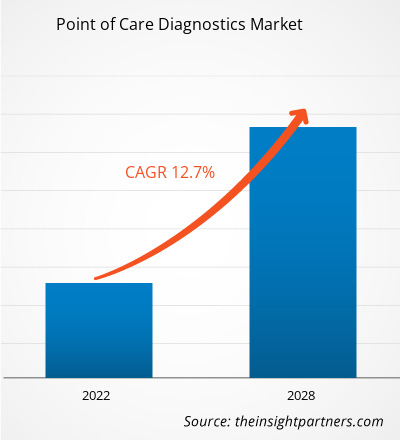
[Research Report] The point of care diagnostics market was valued at US$ 36,000.4 million in 2021 and is projected to reach US$ 82,958.3 million by 2028; it is expected to grow at a CAGR of 12.7% from 2021 to 2028.
Market Insights and Analyst View:
Point-of-care (POC) testing is a term that describes diagnostic testing which is performed in the patient’s unit rather than in the hospital’s central lab or outside reference lab. Point of care tests may be done on blood, urine, swabs from mucosal surfaces, or saliva. In the emergency department (ED), POC testing is typically performed by both ED technicians (EDTs) and nurses. In some jurisdictions, emergency medical services providers use POC tests in pre-hospital settings. Most hospitals have developed a rigorous quality process for their POC testing programs. Point-of-care testing (POCT) is essential for the rapid detection of analytes near to the patient, which facilitates in disease diagnosis, monitoring, and management. It enables quick medical decisions, as the diseases can be diagnosed at a very early stage, and is an important criterion in preventing advanced disease types. This primarily leads to improved health outcomes for patients by enabling the early start of treatment. Recent advancements in biosensors, which is a critical component for point of care diagnostics is directly responsible for the bioanalytical performance of assay.
Growth Drivers and Challenges:
Point-of-care testing (POCT) plays a critical role in diagnosis, treatment, and prevention of infectious diseases. POC testing can be used to detect several major pathogens, including malarial parasites, human immunodeficiency virus (HIV), human papillomavirus (HPV), dengue, Ebola, and Zika viruses; and Mycobacterium tuberculosis (TB bacteria). HIV has infected over 38 million people worldwide; ~85% of this population resides in developing countries, which have a limited availability of clinical diagnostics and antiretroviral therapy (ART) monitoring platforms. To narrow down the diagnostic time, companies are developing POC test kits and reagents. For instance, Food and Drug Administration (FDA) has approved the ARCHITECT HIV Ag/Ab EIA, a fourth-generation assay, introduced by Abbott Laboratories. The increasing incidence of infectious diseases such as HIV is the major global public health issue. In 2019, World Health Organization (WHO) claimed that more than 38 million people were diagnosed with HIV, out of which 1.7 million people were newly diagnosed worldwide, including 62% adults and 54% children. According to a study published by UNAIDS, in 2019, around 690,000 people died because of HIV and associated conditions. As per the European Centre for Disease Prevention and Control, ~2 million people in Europe were infected with HIV in 2019. Moreover, healthcare-associated infections (HAIs) such as central line-associated bloodstream infections and catheter-associated urinary tract infections might affect the patients in hospitals and other healthcare facilities. According to a recent study published on NCBI in 2020, HAI causes around 16 million additional hospitalization days in Europe each year. Moreover, according to the same study, HAI leads to additional financial burden of around US$ 8.5 billion in Europe each year. In addition, according to the Office of Disease Prevention and Health Promotion, 1 out of 25 hospitalizations in the US suffers from HAI at any given time. Such increase in prevalence of infectious diseases is expected to create a demand for point of care tests kits across the world. Such a factor has assisted the growth of point of care diagnostics market and is expected to follow a similar trend during the forecast period.
Product recalls are rising in size and number in the industry, predominantly driven by the increasing complexity of global supply chains, as well as increasingly stringent regulations. In 2018, PTS Panels CHOL+GLU test strips, which are considered as in vitro diagnostic medical devices were recalled in Australia. The cause of recall was related to medical device activity. According to the Department of Health, these devices were manufactured by point-of-care Diagnostics Australia Pty Ltd. Based on the results of internal testing, there was malfunctioning in measurement of the glucose test. This issue may result in the delay in diagnosis of diabetes. Similarly, in April 2020, Trividia Health, Inc. issued a notice for nationwide voluntary recall for its TRUE METRIX AIR Blood Glucose Meter. The company issued this notice due to malfunctioning of measurement units in the glucose meter. Such events are anticipated to hamper the customer confidence toward the use of point of care devices, which will eventually deter the point of care tests market growth.
Moreover, turnaround time for molecular diagnostic tests is important in detecting infectious agents, in determining a patient's ability to metabolize a drug or drug class. This is also essential in detecting minimal residual disease. These applications would benefit from the development of a point-of-care device for nucleic acid extraction, amplification, and detection. The device would have a low cost per test, use a disposable unit use device for all steps in the assay, should be portable, and provide a result that requires no interpretation. The creation of such a device requires miniaturization of current technologies and the use of microfluidics, microarrays, and small-diameter capillary tubes to reduce reagent volumes. This will simplify heat conduction by convection during nucleic acid amplification. This ideal device may be available in in the coming years and will expand the global point of care molecular diagnostics market.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Point of Care Diagnostics Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Point of Care Diagnostics Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Report Segmentation and Scope:
The “Global Point of Care Diagnostics Market” is segmented based on product, end user, prescription mode, and geography. Based on product, the point of care diagnostics market is segmented into glucose monitoring, infectious disease testing, cardiometabolic testing, pregnancy and fertility testing, coagulation testing, tumor/cancer marker, cholesterol testing, urinalysis testing, hematology testing, and other testing products. Based on end user, the point of care diagnostics market is segmented into professional diagnostic centers, research laboratories, homecare, and others. Based on prescription mode, the point of care diagnostics market is bifurcated into OTC testing and prescription-based testing. The point of care diagnostics market based on geography is segmented into North America (the US, Canada, and Mexico), Europe (Germany, France, Italy, the UK, Russia, and Rest of Europe), Asia Pacific (Australia, China, Japan, India, South Korea, and Rest of Asia Pacific), the Middle East & Africa (South Africa, Saudi Arabia, the UAE, and Rest of the Middle East & Africa), and South & Central America (Brazil, Argentina, and Rest of South & Central America).
Segmental Analysis:
Based on product, the point of care diagnostics market is segmented into glucose monitoring, infectious disease testing, cardiometabolic testing, pregnancy and fertility testing, coagulation testing, tumor/cancer marker, cholesterol testing, urinalysis testing, hematology testing, and other testing products. In 2020, the glucose monitoring product segment held the largest share of the market, whereas, infectious disease testing segment is anticipated to register the highest CAGR of 14.1% during the forecast period. The glucose monitoring devices market consists of components such as glucometers, lancets, testing strips and other glucose monitoring devices. In 2020, the testing strips segment held the largest market share among the glucose monitoring devices segments. The glucose monitoring devices or glucose meters are medical devices are used to determine the approximate levels or the concentration of glucose in the blood of the patients living with diabetes. The concentration of the glucose level can be measured by various means such as through testing strips, lancets and others. In the market various market players have introduced their devices for the glucose monitoring that includes Contour Net EZ, Accu-Check Aviva, Abbott FreeStyle, One Touch Verio. They are being offered by player/market participants such as Abbott, F. Hoffman-La Roche Ltd, and others. In addition, due to the rise in the technology advancements, the developments for the new products are offering various benefits to the user. For instance, diamontech GmbH has invented non-invasive glucose monitoring device. The company offers DMT Base, DMT Pocket and DMT Band that helps the diabetes patients for monitoring their glucose level. Therefore, owing to the presence of the various market players that are offering various products and rise in the technological advancements are likely to propel the growth of global point of care diagnostics market.
Infectious diseases are caused by microorganisms, such as bacteria, viruses, fungi, and parasites. Diagnostic test for an infectious agent is used to demonstrate the presence of the infection. Specimens such as urine, blood, sputum, cerebrospinal fluid, and others are subjected to various tests and the disease-causing bacteria and viruses, known as the infectious agents, are identified quickly.
Real-time test results are important for early diagnosis and in determining treatment orientation in medical practice. Point of Care Testing (POCT) provides beneficial and helpful information for diagnosis and treatment through real-time testing at the bedside. Hence, POCT has high utility value in field of infectious diseases. Infectious disease rapid test kits are widely available for a wide variety of prophlogistic pathogen targets, including bacterial, viral, fungal, protozoal, and other disease agents. In January 2023, Cipla Limited announced the launch of Cippoint, a point- of-care testing device. This state-of-the-art device offers a wide range of testing parameters including cardiac markers, diabetes, infectious diseases, fertility, thyroid function, inflammation, metabolic markers, and coagulation markers. The device is CE IVD approved. This essentially indicates that the device is approved by the European In-Vitro Diagnostic Device Directive, thus ensuring reliable testing solutions. With entry in these new segments, Cipla has expanded its product offerings for diagnostics laboratories and their goal is to bridge the current gap in the diagnostic ecosystem in India by providing accurate and reliable test methods at affordable prices.
Based on end use, the point of care diagnostics market is segmented into professional diagnostic centers, research laboratories, homecare, and others. In 2020, the professional diagnostic centers segment held the largest share of the market and is expected to witness a similar trend during the forecast period. However, the homecare segment is anticipated to register the highest CAGR of 13.3% during the forecast period. Patients can easily monitor their glucose levels at home and this essentially helps them in customizing their insulin therapy to manage their diabetes levels. During the pandemic situation, the homecare point of care diagnostics was at boom as this led to fewer hospital visits. Advancements such as telemedicine and technological advancements in point of care testing devices assisted the global market during the COVID-19 crisis.
Regional Analysis:
Based on geography, the point of care diagnostics market is segmented into five key regions: North America, Europe, Asia Pacific, South & Central America, and the Middle East & Africa. In 2022, North America held the largest share of the point of care diagnostics market followed by Europe. Growing prevalence of infectious diseases, government support for advanced diagnostics kits and instruments, and increasing efforts in R&D activities undertaken by the market players operating in the market is expected to aid the regional growth. According to EBSCO Information Services, as of September 2020, more than 1,000 cases of Hepatitis A has been reported in each of these states: Florida, Georgia, Indiana, Kentucky, Ohio, Tennessee, and West Virginia. The number of cases reported since the arrival of COVID-19 has reached 6,358 (as of September 26, 2020), and with some of the current state outbreaks dating back to 2016, 2017, or 2018, the overall number has now exceeded 32,000 cases. Generally, Hepatitis A largely under control in the United States until three years ago, can be easily prevented through the use of a safe and effective vaccine. However, the number of reported cases has taken a dramatic up-turn during the current COVID-19 pandemic. During such situations, the point of care diagnostic solutions proved to be an effective solution for the patients as government organizations issued various rules and regulations to avoid social distancing owing to COVID-19. Lesser hospital visits were one of the main reasons that increased the demand for point of care tests. Such factors is expected to aid the point of care diagnostic tests market during the forecast period.
Point of Care Diagnostics Market Regional Insights
The regional trends and factors influencing the Point of Care Diagnostics Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Point of Care Diagnostics Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Point of Care Diagnostics Market
Point of Care Diagnostics Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 36 Billion |
| Market Size by 2028 | US$ 82.96 Billion |
| Global CAGR (2021 - 2028) | 12.7% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Product
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Point of Care Diagnostics Market Players Density: Understanding Its Impact on Business Dynamics
The Point of Care Diagnostics Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Point of Care Diagnostics Market are:
- F. Hoffmann-La Roche Ltd
- Abbott
- bioMérieux SA
- Johnson & Johnson Services, Inc.
- Nova Biomedical
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Point of Care Diagnostics Market top key players overview
Industry Developments and Future Opportunities:
Various initiatives taken by key players operating in the global point of care diagnostics market are listed below:
- In April 2019, Roche launched VENTANA HER2 Dual ISH companion diagnostic test for breast and gastric cancer patients.
- In April 2019, Bio-Rad Laboratories, Inc. received U.S. Food and Drug Administration (FDA) approval for its BioPlex 2200 Lyme Total Assay. This was an innovative multiplex test method for the diagnosis of Lyme disease.
Competitive Landscape and Key Companies:
Companies such as Roche, Bio-Rad, bioMérieux, Siemens Healthineers, Nova Biomedical, Danaher, PTS Diagnostics, and Abbott has been implementing various strategies that have helped the growth of the company and in turn have brought about various changes in the market. The companies have utilized strategies such as product launch, product up-gradation, patents, expansion of their product portfolio and area expansion for the growth of their organizations. In addition, the companies have invested their efforts to develop products and have received approvals from regulatory bodies such as the US FDA for their products.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Product , Prescription Mode , and End User , and Geography

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, Saudi Arabia, South Africa, South Korea, Spain, United Arab Emirates, United Kingdom, United States
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Point of Care Diagnostics Market
- F. Hoffmann-La Roche Ltd
- Abbott
- bioMérieux SA
- Johnson & Johnson Services, Inc.
- Nova Biomedical
- Siemens AG
- Bio Rad Laboratories Inc.
- BD
- Danaher Corporation
- PTS Diagnostics
 Get Free Sample For
Get Free Sample For