Molecular Diagnostics Segment to Lead Market during 2021–2028
According to our new research study on “Laboratory Developed Test Market Forecast to 2028 – COVID-19 Impact and Global Analysis – by Type and Application,” the market is expected to reach US$ 7,269.3 million by 2028 from US$ 4,524.75 million in 2021; it is expected to grow at a CAGR of 7.0% during 2021–2028. The report highlights trends prevailing in the market and factors driving its growth. The market growth is mainly attributed to factors such as the increasing incidents of cancer and genetic disorders, and large number of product launches. However, changing regulatory landscape is hindering the market growth. For example, in Europe, the In-Vitro Device Regulation (IVDR) compliance will be mandatory for all in vitro diagnostic tests from May 2022; the regulation aims to secure the clinical effectiveness and safety of medical tests, thus transforming the diagnostic industry, which is a great concern for the market players.
Based on type, the laboratory developed test market is segmented into clinical biochemistry, critical care, hematology, microbiology, molecular diagnostics, immunology, and others. The molecular diagnostics segment is expected to held the largest share of the market in 2021.However, the hematology segment is anticipated to register the highest CAGR in the market during the forecast period. The rapid growth of the market for molecular diagnostics is ascribed to the increasing attention toward the development of genetic therapeutics and growing research in human genomics. Technological advantages in molecular tests make them valuable diagnostic tools for detecting a wide range of genetic diseases. For instance, in November 2017, Edico Genome launched the DRAGEN Clinical Genomics Information System (CGIS). It is designed to enable clinical laboratories to develop sequencing-based laboratory developed tests in a quick, simple, and efficient manner.
Impact of COVID-19 Pandemic on Laboratory Developed Test Market:
The COVID-19 pandemic is adversely affecting industries worldwide. The outbreak led to significant disruptions in primary industries such as manufacturing, healthcare, energy & power, electronics & semiconductor, aerospace & defense, and construction in 2020. However, it provided a vital growth opportunity to the laboratory developed test market players. The market experienced exponential growth in demand for PCR-based COVID-19 test kits. The government of many countries is working towards scaling up testing capacity, which is further driving the laboratory developed test market. Many market players have launched products to meet the growing demand. For instance, in August 2020, to address the COVID-19 testing shortage, the US Department of Health and Human Services (HHS) made a statement that effectively rescinded the FDA guidance requiring clinical labs to seek an emergency use authorization (EUA) or submit data to the FDA in conjunction with offering laboratory-developed tests (LDTs).
Quest Diagnostics Incorporated; F. HOFFMANN-LA ROCHE LTD; QIAGEN; Illumina, Inc.,; Eurofins Scientific; Biodesix; Adaptive Biotechnologies; Biotheranostics; Rosetta Genomics Ltd and Guardant Health are among the leading companies in the laboratory developed test market.
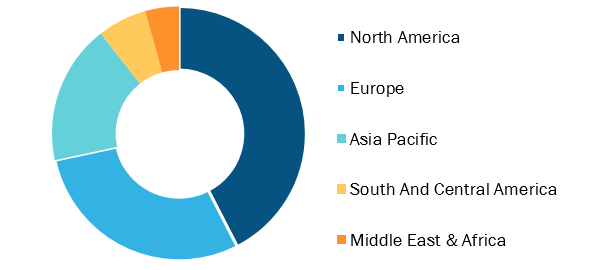
Laboratory Developed Test Market, by Region, 2021 (%)
Laboratory Developed Test Market Forecast and Size by 2028
Download Free Sample
Laboratory Developed Test Market Forecast to 2028 - COVID-19 Impact and Global Analysis By Type (Clinical Biochemistry, Critical Care, Hematology, Microbiology, Molecular Diagnostics, Immunology, and Others) and Application (Academic Institutes, Clinical Research Organizations, Hospitals Laboratory, Specialty Diagnostic Centers, and Others)
Laboratory Developed Test Market Forecast and Size by 2028
Download Free SampleLaboratory Developed Test Market Forecast to 2028 - COVID-19 Impact and Global Analysis By Type (Clinical Biochemistry, Critical Care, Hematology, Microbiology, Molecular Diagnostics, Immunology, and Others) and Application (Academic Institutes, Clinical Research Organizations, Hospitals Laboratory, Specialty Diagnostic Centers, and Others)
Based on type, the laboratory developed test market is segmented into clinical biochemistry, critical care, hematology, microbiology, molecular diagnostics, immunology, and others. Based on Application, the laboratory developed test market is segmented into academic institutes, clinical research organizations, hospitals laboratory, specialty diagnostic centers, and others. On the basis of geography, the laboratory developed test market is segmented into North America (the US, Canada, and Mexico), Europe (the UK, Germany, France, Italy, Spain, and Rest of Europe), Asia Pacific (China, Japan, India, Australia, South Korea, and Rest of Asia Pacific), the Middle East & Africa (the UAE, Saudi Arabia, South Africa, and Rest of the Middle East & Africa), and South and Central America (Brazil, Argentina, and the Rest of South and Central America).
Contact Us
Phone: +1-646-491-9876
Email Id: sales@theinsightpartners.com