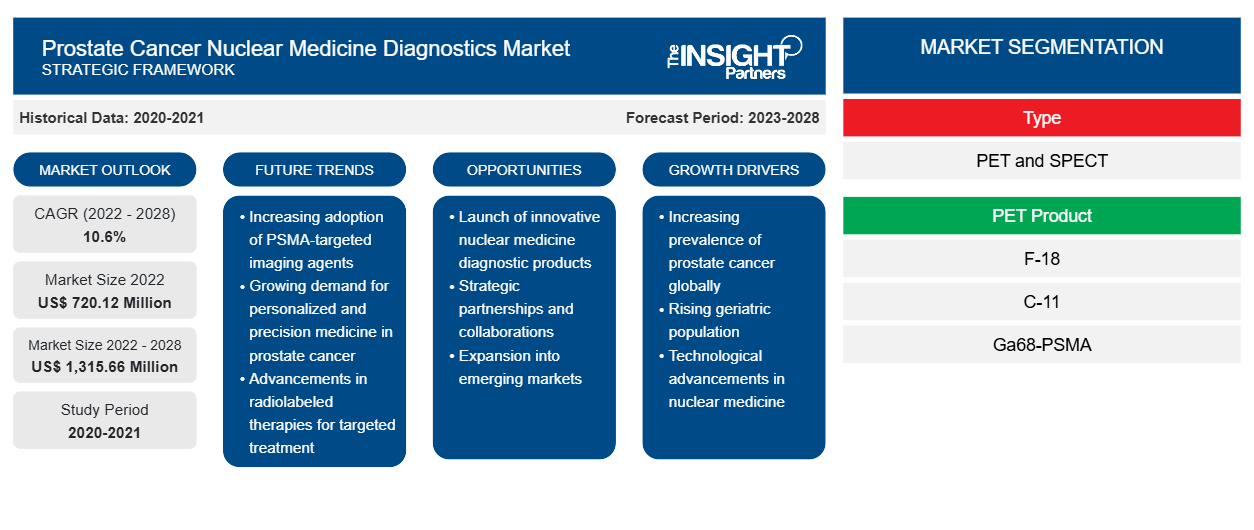
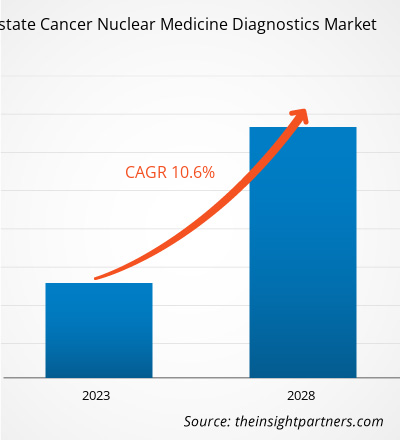
The prostate cancer nuclear medicine diagnostics market is expected to grow from US$ 720.12 million in 2022 to US$ 1,315.66 million by 2028. It is estimated to grow at a CAGR of 10.6% from 2022 to 2028.
Prostate cancer affects the prostate glands in males. Prostate cancer is common cancer following skin cancer in males. Some common determinants responsible for the start of prostate cancer are family history, old age, and race. Nuclear medicine is imaging that needs radioactive materials. It is a helpful method to identify and treat prostate cancer and helps radiologists conclude the stage of cancer.
The prostate cancer nuclear medicine diagnostics market is analyzed into type, PET product, end user, and geography. By geography, the market is broadly segmented into North America, Europe, Asia Pacific, the Middle East & Africa, and South & Central America. The prostate cancer nuclear medicine diagnostics market report offers insights and in-depth analysis of the market, emphasizing parameters, such as market trends, market dynamics, and the competitive analysis of the globally leading market players.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Prostate Cancer Nuclear Medicine Diagnostics Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Prostate Cancer Nuclear Medicine Diagnostics Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Market Insights
Surging Demand for Early and Precise Diagnosis of Prostate Cancer to Drive Prostate Cancer Nuclear Medicine Diagnostics Market
The rise in PSA levels increases the risk of prostate cancer. PSA levels in the blood are measured in nanograms per milliliter (ng/mL) units. There is no definitive cutoff point that can determine if a man has prostate cancer or not. When assessing whether a man needs further testing, many doctors choose a PSA cutoff of 4 ng/mL or above, while others may advocate starting at a lower level, such as 2.5 or 3. Therefore, testing PSA levels in a man's blood can detect prostate cancer early.
A digital rectal exam (DRE) is another approach for detecting prostate cancer. The doctor uses a gloved, lubricated finger to feel the prostate gland during the exam. Regular screening with a well-validated biomarker could lead to quicker detection of localized disease. Furthermore, distinguishing between low-grade and high-grade disease is critical to avoid unnecessary biopsies, undertreatment of serious disease, and overtreatment of indolent disease.
Following are a few examples of companies that launched new products developments and product approvals:
- In April 2022, Telix announced that its prostate cancer imaging agent, Illuccix (kit for the preparation of gallium Ga 68 gozetotide), also known as 68Ga-PSMA-11 injection, will be commercially available in the US.
- In March 2022, Novartis announced that the FDA approved Pluvicto (lutetium Lu 177 vipivotide tetraxetan) (formerly known as 177Lu-PSMA-617) for the treatment of adult patients with prostate-specific membrane antigen–positive metastatic castration-resistant prostate cancer (PSMA-positive mCRPC) that has spread to other parts of the body (metastatic).
- In March 2022, the FDA approved a complementary diagnostic imaging agent, Locametz, after radiolabeling with gallium-68 to identify PSMA-positive lesions. After radiolabeling, this imaging agent may be used to identify PSMA-positive lesions in adult patients with mCRPC through a positron emission tomography scan.
Such product developments by market players are driving the prostate cancer nuclear medicine diagnostics market.
Type Insights
Based on type, the global prostate cancer nuclear medicine diagnostics market is segmented into SPECT and PET. The PET segment held the largest share of the market in 2021. Moreover, the same segment is anticipated to register the highest CAGR in the market during the forecast period. The adoption of PET as a diagnostic tool is significantly increasing, as it offers higher accuracy than other diagnostic techniques. Accuracy in diagnosis has a direct impact on decision-making and treatment monitoring processes. The increasing demand for these diagnostic procedures is expected to fuel the growth of the prostate cancer nuclear medicine diagnostics market during the forecast period.
Services Insights
Based on PET products, the global prostate cancer nuclear medicine diagnostics market is segmented into F-18, C-11, and Ga68-PSMA. The F-18 segment is likely to hold the largest share of the market in 2022. However, the Ga68-PSMA segment is anticipated to register the highest CAGR in the market during the forecast period. Ga68-PSMA is a non-invasive diagnostic approach that uses enhanced prostate-specific membrane antigen (PSMA) expression to imaging prostate cancer. PSMA is a transmembrane protein found throughout the prostate. PSMA expression is upregulated in a variety of cancers, but prostate cancer has the highest levels. Furthermore, PSMA expression has a predictive value for illness outcomes. PET produces semi-quantitative pictures that enable non-invasive measurement of PSMA expression by measuring the three-dimensional distribution of Ga68-PSMA.
Partnerships and collaborations are highly adopted strategies by the global prostate cancer nuclear medicine diagnostics market players. A few of the recent key market developments are listed below:
- In April 2022, Telix announced a partnership with Avanço and THP to distribute Telix's prostate cancer investigational imaging product for the Portuguese and Austrian, Czech Republic, and Slovak Republic markets.
- In August 2022, Telix released details of two ancillary studies under the ProstACT program, including a Phase II study collaborating with GenesisCare.
- In February 2021, Theragnostics announced a research agreement with Essen University Hospital to investigate THG-008 for PET imaging in several cancers as radiolabeled PARP inhibitors have the potential to improve patient survival in cancer.
The outbreak of the COVID-19 pandemic situation has shown some favorable scenarios for players operating in the prostate cancer nuclear medicine diagnostics market. Various people across the North American region are affected by COVID 19. the US is a highly affected country in the North American region. The infection has severely affected the geriatric population in the country, causing various complications and leading to the death of a large population. For instance, As of May 16, 2022, The US report 84,230,829 COVID cases with the death of 1,026,670 people. In addition, chronic wounds or non-healing wounds are mostly seen in older people and take longer to heal. Therefore, prostate cancer nuclear medicine diagnostics and proper care are required to prevent chronic cases of infection and health conditions.
Furthermore, due to the outbreak of COVID-19, various hospitals were engaged in treating the affected people. The COVID-19 epidemic resulted in a significant drop in cancer screening. However, the epidemic has resulted in a total screening deficiency in the United States. COVID-19-related delays in cancer screenings are thought to be causing additional excess fatalities that are directly linked to the epidemic. Prostate biopsy and PC diagnosis rates fell during the COVID-19 pandemic, especially during the outbreak's peak.
Additionally, before the pandemic, imaging services in Europe, mainly the UK, suffered from chronic underinvestment with a lack of imaging acquisition and reporting capacity. For example, this was related to a significant workforce and equipment shortage. But with new IPC measures, the particular social distancing of 2m in all healthcare settings, these shortages have met CT capacity at the time of writing about 40-70% of pre-COVID levels. Also, MRI is slightly better, accounting for 80%. Further, the imaging services are working hard to regain momentum by restoring capacity and recovering patient confidence. It remains clear that fundamental changes can only be made with significant investment for addressing the chronic underfunding of radiologists, radiographers, and imaging equipment.
Prostate Cancer Nuclear Medicine Diagnostics Market Regional Insights
Prostate Cancer Nuclear Medicine Diagnostics Market Regional Insights
The regional trends and factors influencing the Prostate Cancer Nuclear Medicine Diagnostics Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Prostate Cancer Nuclear Medicine Diagnostics Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
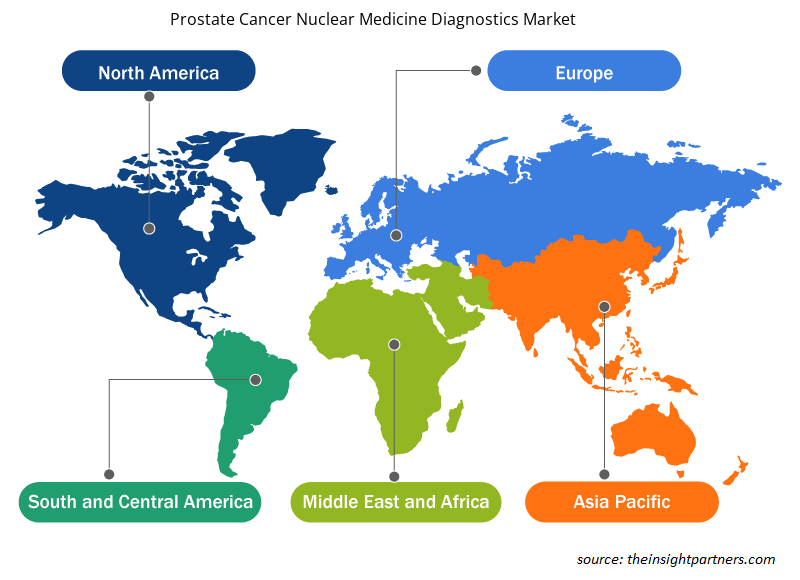
- Get the Regional Specific Data for Prostate Cancer Nuclear Medicine Diagnostics Market
Prostate Cancer Nuclear Medicine Diagnostics Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 720.12 Million |
| Market Size by 2028 | US$ 1,315.66 Million |
| Global CAGR (2022 - 2028) | 10.6% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2028 |
| Segments Covered |
By Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Prostate Cancer Nuclear Medicine Diagnostics Market Players Density: Understanding Its Impact on Business Dynamics
The Prostate Cancer Nuclear Medicine Diagnostics Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Prostate Cancer Nuclear Medicine Diagnostics Market are:
- Blue Earth Diagnostics Limited
- ImaginAB
- Curium
- Jubilant Radiopharma
- NCM-USA LLC
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Prostate Cancer Nuclear Medicine Diagnostics Market top key players overview
Prostate Cancer Nuclear Medicine Diagnostics– Market Segmentation
The global prostate cancer nuclear medicine diagnostics market is analyzed on the basis of type, PET product, end user, and geography. In terms of type, the market is bifurcated into PET and SPECT. Based on PET product, the market is segmented into F-18, C-11, and Ga68-PSMA. Based on end user, the market is segmented into hospitals, clinics, and others. Geographically, the prostate cancer nuclear medicine diagnostics market is segmented into North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America.
Company Profiles
- Blue Earth Diagnostics Limited
- ImaginAb
- Curium
- Jubilant Radiopharma
- NCM-USA LLC
- ABX advanced biochemical compounds GmbH
- Telix Pharmaceuticals Ltd.
- Novartis AG
- Theragnostics
- Lantheus Medical Imaging, Inc.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Type, PET Product, and End User

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, Saudi Arabia, South Africa, South Korea, Spain, United Arab Emirates, United Kingdom, United States
Frequently Asked Questions
The global prostate cancer nuclear medicine diagnostics market on region, is segmented into North America, Europe, Asia Pacific, Middle East & Africa, and the South & Central America. In 2021, North American region held largest market share. However, Asia Pacific region is estimated to grow at the fastest CAGR of 11.2% during the forecast period
The global prostate cancer nuclear medicine diagnostics market based on type, is segmented into SPECT and PET. In 2021, PET segment held largest market share and the segment is estimated to grow at the fastest CAGR of 10.8% during the forecast period.
The high cost related to the prostate cancer diagnostics and treatment is expected to restrict the market growth during the forecast period
The prostate cancer nuclear medicine diagnostics market majorly consists of the players such as Blue Earth Diagnostics Limited, ImaginAB, Curium, Jubilant Radiopharma, NCM-USA LLC, ABX Advanced Biochemical Compounds GmbH, Telix Pharmaceuticals Ltd., Novartis AG, Theragonostics, and Lantheus Medical Imaging Inc.
Factors such as the rising prevalence of prostate cancer and surging demand for early and precise diagnosis of prostate cancer.
Prostate cancer affects the prostate glands in males. Prostate cancer is common cancer following skin cancer in males. Some common determinants responsible for prostate cancer are family history, old age, and race. Nuclear medicine is imaging that needs radioactive materials. It is a helpful method to identify and treat prostate cancer and helps radiologists conclude the stage of cancer. Prostate cancer (PCa) is the fourth most common cancer across the US in terms of occurrence.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Prostate Cancer Nuclear Medicine Diagnostics Market
- Blue Earth Diagnostics Limited
- ImaginAB
- Curium
- Jubilant Radiopharma
- NCM-USA LLC
- ABX Advanced Biochemical Compounds GmbH
- Telix Pharmaceuticals Ltd.
- Novartis AG
- Theragonostics
- Lantheus Medical Imaging Inc.
 Get Free Sample For
Get Free Sample For