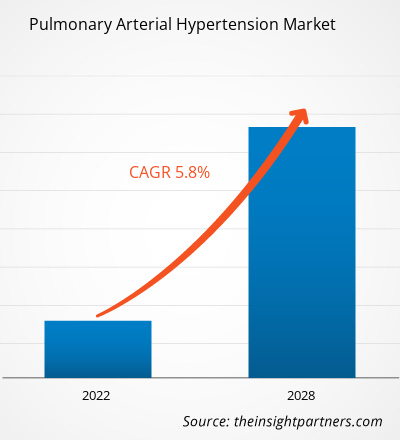
The pulmonary arterial hypertension market is expected to grow from US$ 7,369.89 million in 2021 to US$ 10,889.08 million by 2028; it is estimated to grow at a CAGR of 5.8% from 2022 to 2028.
The pulmonary arterial hypertension market is segmented on the basis of drug, type, route of administration, distribution channel, and geography. The report offers insights and in-depth analysis of the pulmonary arterial hypertension market, emphasizing various parameters such as dynamics, trends, and opportunities of the market and competitive landscape analysis of leading market players across various regions. It also includes the impact analysis of COVID–19 pandemic on pulmonary arterial hypertension market across the regions.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Pulmonary Arterial Hypertension Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Pulmonary Arterial Hypertension Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Market Insights
Rising Approvals of Pulmonary Arterial Hypertension Drugs Drives Market Growth
- In September 2022, Toray Industries, Inc. received approval from China’s National Medical Products Administration (NMPA) for Careload tablets to treat pulmonary arterial hypertension (PAH). Careload is an orally administered sustained-release formulation of beraprost sodium, a prostacyclin derivative (PGI2).
- In May 2022, United Therapeutic Corporation received US Food and Drug Administration (FDA) approval for Tyvaso DPI (treprostinil) inhalation powder to treat pulmonary hypertension associated with interstitial lung disease (PH-ILD; WHO Group 3) and pulmonary arterial hypertension (PAH; WHO Group 1) to enhance exercise ability.
- In November 2021, the US FDA granted tentative approval for Yutrepia (treprostinil) inhalation powder. Yutrepia is indicated to treat PAH to improve exercise ability in adult patients with New York Heart Association (NYHA) Functional Class II-III symptoms.
- In July 2021, The Janssen Pharmaceutical Companies of Johnson & Johnson received FDA approval for UPTRAVI (selexipag) injection for intravenous (IV) to treat PAH; WHO Group I in adult patients with WHO functional class (FC) II–III, who are temporarily unable to take oral therapy.
- In August 2021, Aerami Therapeutics, Inc. announced that the FDA granted the company orphan drug designation for imatinib to treat PAH patients. AER– 901, a drug-device combination product candidate for inhaled imatinib for the treatment of PAH, is in a Phase 1 trial with completion targeted for the end of 2021.
The rising approvals of pulmonary arterial hypertension drugs across the world boost the growth of the pulmonary arterial hypertension market.
Drug-Based Insights
Based on drug, the pulmonary arterial hypertension market is segmented into endothelin receptor antagonists (ERAs), prostacyclin and prostacyclin analogs, sGC stimulators, and pde-5 dipsticks. The prostacyclin and prostacyclin analogs segment held the largest market share in 2021 and is anticipated to register the highest CAGR during 2022–2028. Prostacyclin is a small lipid eicosanoid molecule, also known as prostaglandin, I-2 (PGI-2), produced by endothelial cells and acts locally, inhibiting platelet activation and causing vasodilation. Prostacyclin dilates blood vessels, improves blood flow by preventing the proliferation of vascular smooth muscle cells, and prevents blood clotting by inhibiting platelet aggregation. Prostacyclin analogs have been developed as therapies for PAH. PAH prostacyclin analogs are synthetic forms of prostacyclin, a natural substance produced in the body. Patients affected by PAH tend to have reduced prostacyclin levels, leading to blood vessel constriction and hypertension. PAH prostacyclin analogs supplement the requirement and mimic the action of natural prostacyclin to reduce pulmonary hypertension and reduce associated symptoms.
Type-Based Insights
Based on type, the pulmonary arterial hypertension market is bifurcated into branded and generics. The branded segment held a larger market share in 2021 and is expected to register a higher CAGR during the forecast period. A drug company sells a branded drug under a specific name or trademark and that is protected by a patent. Brand-name drugs may be available by prescription or over the counter. Riociguat, sold under the brand name Adempas, is Bayer's medication stimulator of soluble guanylate cyclase. It is used to treat two forms of pulmonary hypertension—chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension.
Route of Administration-Based Insights
Based on route of administration, the pulmonary arterial hypertension market is segmented into oral, intravenous/subcutaneous, and inhalational. The oral segment held the largest market share in 2021 and is anticipated to register the highest CAGR during the forecast period. Orally administered agents used for the treatment of symptomatic, moderate-to-severe PAH include sildenafil and the endothelin (ET) receptor antagonists (ERAs), bosentan and sitaxentan (sitaxsentan), and abrisentan oral ET(A) receptor-selective ERA, with higher ET receptor affinity than bosentan. Moreover, new oral drugs for treating PAH include macitentan, riociguat, and treprostinil. Ambrisentan is an endothelin receptor antagonist indicated for treating PAH; WHO Group 1 and delay clinical worsening. In combination with tadalafil, ambrisentan is indicated to reduce the risks of disease progression and hospitalization for worsening PAH, and to improve exercise ability.
Distribution Channel-Based Insights
Based on distribution channel, the pulmonary arterial hypertension market is segmented into hospital pharmacies and clinics, online pharmacies, and retail pharmacies. The hospital pharmacies and clinics segment held the largest market share in 2021 and is anticipated to register the highest CAGR during the forecast period. Hospital pharmacy strives to continuously maintain and improve patients' medication management and pharmaceutical care to the highest standards in a hospital setting. Pharmacists in this field are responsible for dispensing medications, quality testing, formulating and re-formulating dosage forms, monitoring, reporting drug safety, and preparing budgets for medications.
The pulmonary arterial hypertension market players adopt organic strategies, such as product launch and expansion, to expand their footprint and product portfolio across the world and meet the growing demand. Inorganic growth strategies witnessed in the market are partnerships and collaborations. These growth strategies have allowed the market players to expand their businesses and enhance their geographic presence. Additionally, acquisitions, partnerships, and other growth strategies help strengthen the company’s customer base and increase its product portfolio.
- In September 2022, Lupin launched Sildenafil for oral suspension in the US market with the US FDA approval. The drug is a generic version of Revatio for oral suspension of Viatris Specialty LLC. Sildenafil for oral suspension treats PAH.
- In February 2021, Aerami Therapeutics, Inc. signed an exclusive license and development agreement with Hangzhou Chance Pharmaceuticals Co. Ltd. (Chance) to develop and commercialize Aerami’s drug-device combination product candidate (“AER-901”) for the treatment of PAH in mainland China, Hong Kong, Macau, and Taiwan. Under the agreement, Chance is responsible for the overall development and commercialization of AER-901 for PAH in the territory.
Pulmonary Arterial Hypertension Market Regional Insights
Pulmonary Arterial Hypertension Market Regional Insights
The regional trends and factors influencing the Pulmonary Arterial Hypertension Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Pulmonary Arterial Hypertension Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Pulmonary Arterial Hypertension Market
Pulmonary Arterial Hypertension Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 7.37 Billion |
| Market Size by 2028 | US$ 10.89 Billion |
| Global CAGR (2021 - 2028) | 5.8% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Drugs
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Pulmonary Arterial Hypertension Market Players Density: Understanding Its Impact on Business Dynamics
The Pulmonary Arterial Hypertension Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Pulmonary Arterial Hypertension Market are:
- Johnson & Johnson
- Gilead Sciences Inc
- United Therapeutics Corp
- Bayer AG
- Aerami Therapeutics Holdings Inc
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Pulmonary Arterial Hypertension Market top key players overview
Company Profiles
- Johnson & Johnson
- Gilead Sciences Inc
- United Therapeutics Corp
- Bayer AG
- Aerami Therapeutics Holdings Inc
- Novartis AG
- GSK Plc
- Lupin Ltd
- Pfizer Inc
- Teva Pharmaceutical Industries Ltd.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset


- Pharmacovigilance and Drug Safety Software Market
- Smart Grid Sensors Market
- Railway Braking System Market
- Health Economics and Outcome Research (HEOR) Services Market
- Long Read Sequencing Market
- Third Party Logistics Market
- Wheat Protein Market
- Wire Harness Market
- HVAC Sensors Market
- Flexible Garden Hoses Market

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Drugs, Type, Route of Administration, and Distribution Channel

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, Saudi Arabia, South Africa, South Korea, Spain, United Arab Emirates, United Kingdom, United States
Frequently Asked Questions
The global pulmonary arterial hypertension based on regions is segmented into North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America. In 2021, North America held the largest market share. However, the Asia Pacific region is estimated to grow at the fastest CAGR of 6.5% during the forecast period.
Based on drugs, the pulmonary arterial hypertension market is segmented into prostacyclin and prostacyclin analogs, endothelin receptor antagonists (ERAs), sGC stimulators, and pde-5 dipsticks. In 2021, the prostacyclin and prostacyclin analogs segment accounted for a larger market share. Also, the same segment is anticipated to register a higher CAGR during the forecast period as the PAH prostacyclin analogs are used as medications to treat pulmonary arterial hypertension (PAH), a condition of high blood pressure in the arteries that carry deoxygenated blood from the heart to the lungs.
Pulmonary arterial hypertension majorly consists of the players, such as Johnson & Johnson, Gilead Sciences Inc, United Therapeutics Corp, Bayer AG, Aerami Therapeutics Holdings Inc, Novartis AG, GSK Plc, Teva Pharmaceutical Industries Ltd, Lupin Ltd, and Pfizer Inc.
The factors driving the growth of pulmonary arterial hypertension are the growing incidence of pulmonary arterial hypertension and the rising approvals of pulmonary arterial hypertension drugs.
The side effects of drugs used for treating pulmonary arterial hypertension are expected to restrict the pulmonary arterial hypertension market growth during the forecast period.
Pulmonary arterial hypertension (PAH) is a rare, progressive disorder characterized by high blood pressure (hypertension) in the lungs' arteries. The increased pressure in the vessels may be caused by an obstruction in the lung's small arteries for various reasons. The exact cause of PAH is unknown, and although treatable, there is no permanent cure for the disease. PAH usually affects women between the ages of 30-60. However, individuals with PAH may go years without a diagnosis, either because the symptoms may be mild, nonspecific, or only present during demanding exercise.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Butterfly Needles Market
- Johnson & Johnson
- Gilead Sciences Inc
- United Therapeutics Corp
- Bayer AG
- Aerami Therapeutics Holdings Inc
- Novartis AG
- GSK Plc
- Teva Pharmaceutical Industries Ltd
- Lupin Ltd
- Pfizer Inc
 Get Free Sample For
Get Free Sample For