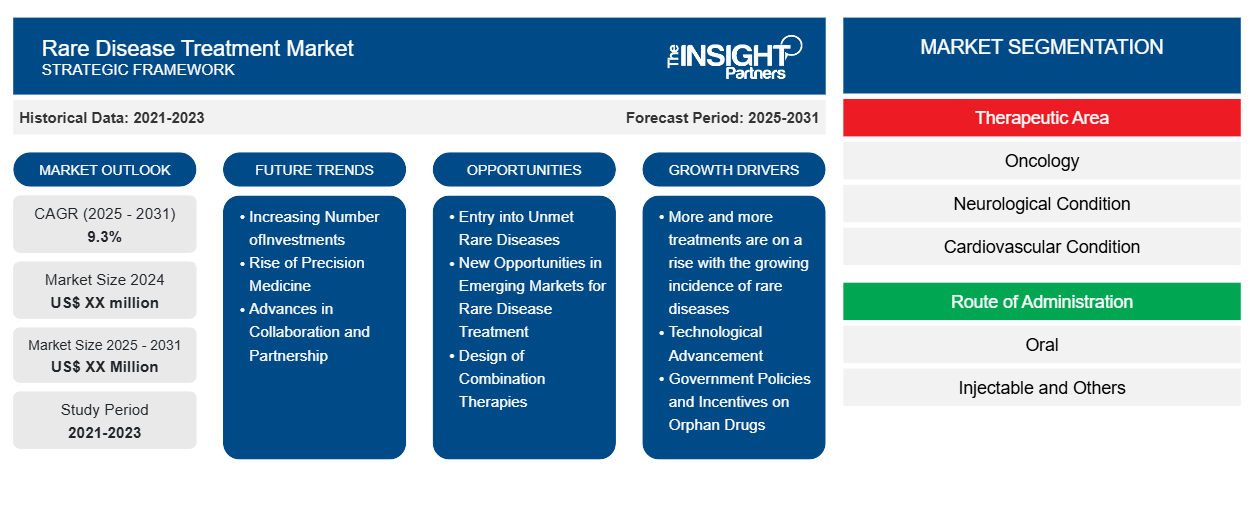
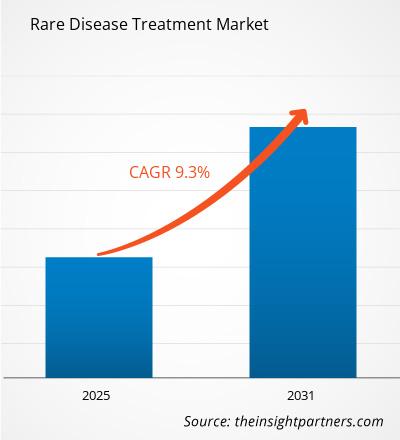
The Rare Disease Treatment Market is expected to register a CAGR of 9.3% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The report is segmented By Therapeutic Area (Oncology, Neurological Condition, Cardiovascular Condition, Musculoskeletal Condition, Infectious Diseases, Others), Route of Administration (Oral, Injectable and Others), Drug Type (Biologics, Small Molecule and Biosimilars) and by Distribution Channel (Online Pharmacy, Specialty Pharmacy and Hospital Pharmacy),
The global analysis is further broken-down at regional level and major countries. The Report Offers the Value in USD for the above analysis and segments.
Purpose of the Report
The report Rare Disease Treatment Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
Rare Disease Treatment Market Segmentation
Therapeutic Area
- Oncology
- Neurological Condition
- Cardiovascular Condition
- Musculoskeletal Condition
- Infectious Diseases
- Others
Route of Administration
- Oral
- Injectable and Others
Drug Type
- Biologics
- Small Molecule and Biosimilars
Distribution Channel
- Online Pharmacy
- Specialty Pharmacy and Hospital Pharmacy
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Rare Disease Treatment Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Rare Disease Treatment Market Growth Drivers
- More and more treatments are on a rise with the growing incidence of rare diseases: The success rate in finding rare conditions in healthcare has led to better diagnosis in patients, thereby continuing to grow the market for specialized therapies. Among such areas of great strength in genetics, autoimmunity, and neurological disorders that require specific treatment.
- Technological Advancement: Advancements in biotechnology and gene editing technologies, for example, CRISPR technologies, have changed the treatment approach of rare diseases. Advanced personal therapy, which is essentially a gene therapy targeting the cause of genetic disorders, motivates more advanced treatments. Pharmaceutical companies and investors responding to the demand therefore increase market size over the treatment of rare diseases.
- Government Policies and Incentives on Orphan Drugs: World governments are writing legislation to encourage the development of drugs for rare diseases. In the United States, tax breaks, market exclusivity, and grants are inducements under the Orphan Drug Act that may be given to companies developing drugs that treat rare disorders. Other region's programs and in the EU made the investment in rare disease drugs more profitable
Rare Disease Treatment Market Future Trends
- Increasing Number ofInvestments: An increasing number of investments in gene and cell therapies are being made within the rare disease treatment landscape, and this area will continue to see growth, especially in genetic diseases such as cystic fibrosis or Duchenne muscular dystrophy. In the case of gene and cell therapies, there is a possibility of cure or long-term management through these treatments by addressing the genetic causes of these diseases. That is one particular trend observed in the rare disease space.
- Rise of Precision Medicine: Now one of the newest decisive trends in the treatments of rare diseases is precision medicine. With the revolution in genomics-and especially the identification of biomarkers-the possibility of a treatment targeted to genetic profiles can represent effective and focused therapies, especially for rare and complex diseases, achieving thereby better patient outcomes and reducing side effects of drugs when applied precisely for specific genetic mutations.
- Advances in Collaboration and Partnership: Pharmaceutical companies collaborate with academia, biotechs, and patient advocacy groups to identify the potential new treatments of the rare diseases. In this regard, such collaborations share resources and expertise for faster development of new therapies. Also, these models of collaboration will thrive in the rare disease space that continues with public-private partnership arrangements.
Rare Disease Treatment Market Opportunities
- Entry into Unmet Rare Diseases: Rare diseases constitute a vast area where medicinal companies can focus their efforts since most such diseases do not have an effective treatment or are effective only to a modest extent. Companies can aim their attention at unmet needs in areas like rare cancers, neuromuscular diseases, and rare metabolic disorders to fill gaps in current therapies and exploitation of lucrative though niche markets with high demand for innovative treatments.
- New Opportunities in Emerging Markets for Rare Disease Treatment: With benefits in access to healthcare in emerging markets now greatly visible, demand in the treatments of rare diseases in regions such as Asia-Pacific, Latin America, and the Middle East is on a rise. Companies have an enormous opportunity in considering a move into these regions due to increasing awareness and diagnostic abilities of novel therapies going to be needed.
- Design of Combination Therapies: Since most of the rare diseases are complex perhaps such conditions may need multi-faceted treatment processes. This would provide a window to design combination therapies, which impact the rare disease on multiple aspects simultaneously and enhance the result of treatment. This is applicable very much so in conditions such as rare cancers, autoimmune diseases, and genetic disorders, where multi-drug approaches would be in place.
Rare Disease Treatment Market Regional Insights
The regional trends and factors influencing the Rare Disease Treatment Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Rare Disease Treatment Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Rare Disease Treatment Market
Rare Disease Treatment Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ XX million |
| Market Size by 2031 | US$ XX Million |
| Global CAGR (2025 - 2031) | 9.3% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Therapeutic Area
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Rare Disease Treatment Market Players Density: Understanding Its Impact on Business Dynamics
The Rare Disease Treatment Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Rare Disease Treatment Market are:
- F. Hoffmann-La Roche Ltd.
- Pfizer, Inc.
- PTC Therapeutics
- Novartis AG
- Takeda Pharmaceutical Company
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Rare Disease Treatment Market top key players overview
Key Selling Points
- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the Rare Disease Treatment Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the Rare Disease Treatment Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
This text is related
to segments covered.

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
F. Hoffmann-La Roche Ltd., Pfizer, Inc., PTC Therapeutics, Novartis AG, Takeda Pharmaceutical Company, Bayer AG, AbbVie Inc., Merck & Co. Inc., and Bristol Myers Squibb
The market is expected to grow at a CAGR of 10.2%
The Rare Disease Treatment Market is estimated to witness a CAGR of 9.3% from 2023 to 2031
Asia Pacific region dominated the Rare Disease Treatment Market in 2023
North America region dominated the Rare Disease Treatment Market in 2023
Asia Pacific is estimated to grow at the highest CAGR over the forecast year (2023: 2031)
Trends and growth analysis reports related to Life Sciences : READ MORE..
- F. Hoffmann-La Roche Ltd.
- Pfizer, Inc.
- PTC Therapeutics
- Novartis AG
- Takeda Pharmaceutical Company
- Bayer AG
- AbbVie Inc.
- Merck & Co. Inc.
- Bristol Myers Squibb
 Get Free Sample For
Get Free Sample For