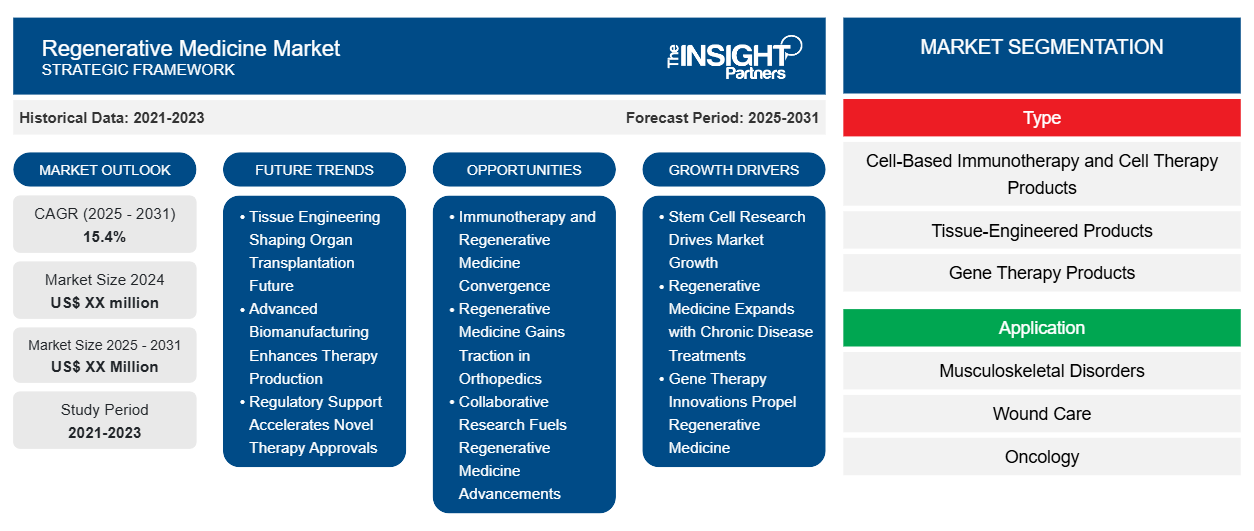
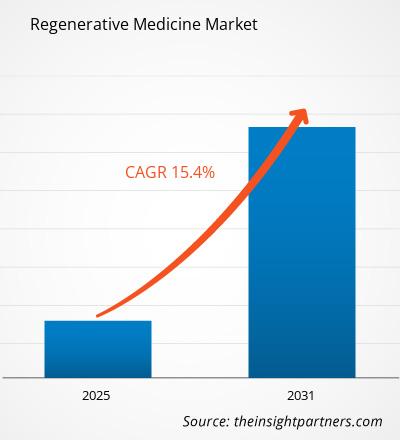
The Regenerative Medicine Market is expected to register a CAGR of 15.4% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The Regenerative Medicine Market report covers analysis by Type (Cell-Based Immunotherapy and Cell Therapy Products, Tissue-Engineered Products, and Gene Therapy Products) and Application (Musculoskeletal Disorders, Wound Care, Oncology, Ocular Disorders, and Diabetes). The research report provides a global and regional overview of the market.
Purpose of the Report
The report Regenerative Medicine Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
Regenerative Medicine Market Segmentation
Type
- Cell-Based Immunotherapy and Cell Therapy Products
- Tissue-Engineered Products
- Gene Therapy Products
Application
- Musculoskeletal Disorders
- Wound Care
- Oncology
- Ocular Disorders
- Diabetes
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Regenerative Medicine Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Regenerative Medicine Market Growth Drivers
- Stem Cell Research Drives Market Growth: Advances in research regarding stem cells, which have been revolutionizing treatments of degenerative diseases, drive this market. The increasing demand for therapies based on the potential use of stem cells to repair or replace faulty tissues and issues is trending upward in most markets. Recent breakthroughs in tissue engineering and gene editing have further opened the door to the international market to increase market share for advanced medicines. Market reports suggest that the growth prospects of healthcare are motivated by stem cell therapies, which will drive the healthcare market.
- Regenerative Medicine Expands with Chronic Disease Treatments: Regenerative medical therapy happens to be a related area, wherein chronic diseases such as diabetes, cardiovascular disorders, or neurodegenerative conditions have been increasing and doing so progressively. Regenerative medicine has the potential to restore damaged tissues, potentially changing patient outcomes dramatically. An analysis of the market, therefore, shows that this trend is fueling market growth in that healthcare systems and pharmaceutical companies invest much in research and clinical trials. Global markets are, thus, experiencing expansion supported by these emerging therapeutic solutions.
- Gene Therapy Innovations Propel Regenerative Medicine: Gene therapy innovations hold a massive mandate under the umbrella of growth for the global regenerative medicine market, with transformative power in the treatment of genetic disorders. Breakthroughs in CRISPR and other gene-editing technologies are advancing personal medicine. These breakthroughs are said to dictate the trend of markets and further propel business growth. Therefore, the market size will grow further as companies keep investing in research and development to obtain the upper hand in having a more significant portion of the global market as the regulatory approvals for gene therapies continue to rise.
Regenerative Medicine Market Future Trends
- Tissue Engineering Shaping Organ Transplantation Future: The emerging tissue engineering trend is shaping the future of regenerative medicine, especially in organ transplantation. Key players are investing in the ability to develop bioengineered organs due to the lack of availability worldwide of donor organs. SWOT analysis marks possible disruptive innovation with challenges in scalability and ethics debates. The PEST analysis thus involves government funding and positive public perception, while the market strategies involve long-term R&D in further improving bioengineering techniques and commercial viability.
- Advanced Biomanufacturing Enhances Therapy Production: Advanced biomanufacturing techniques are revolutionizing the production of cell-based therapies and tissue-engineered constructs regenerative medicine products. The leaders in this market innovate with new market strategies to make their production much more efficient and scalable. The SWOT analysis reveals that this strength in cutting down costs and streamlining processes also becomes a weakness by degrading the product and failing to meet the set regulatory standards. The PEST analysis reflects that the government is coming up with necessary initiatives that are sustaining the growth within the industry of biomanufacturing so that the market is dynamic where technological advances lead to developments in regenerative solutions.
- Regulatory Support Accelerates Novel Therapy Approvals: The emerging regenerative medicine market requires increased regulatory support to further the approval process of novel therapies. The governments are introducing accelerated review programs for regenerative therapies and challenging the dominant players to pursue aggressive market strategies that would accelerate product launches. In a SWOT analysis, better regulatory frameworks again augment entry opportunities in the market although stringent compliance terms push up the disadvantages. The PEST analysis reveals that favorable regulations favor innovation, pushing research as well as clinical applications of this sector more dynamically.
Regenerative Medicine Market Opportunities
- Immunotherapy and Regenerative Medicine Convergence: Immunotherapy and regenerative medicine are converging, opening new avenues for growth. More than 30% of the North American clinical trials conducted in 2023 applied these approaches in combination to enhance therapy outcomes, especially in oncology. It provides an opportunity for large numbers of companies looking for differentiation in the competitive landscape. The Asia-Pacific and Europe geographies also study this type of convergence, which drives further research and investment. The integration of these fields will redefine all future treatment modalities across the globe.
- Regenerative Medicine Gains Traction in Orthopedics: Increasingly, regenerative medicine is being increasingly applied in the field of orthopedics and it provides an enormous growth opportunity. In 2023, fifty percent of North American clinics involved in the treatment of orthopedic disorders adopted regenerative treatments including stem cell therapy. Competitive analysis indicates growing interest in this domain within Europe and Latin America wherein there is an increased tendency to demand regenerative therapies due to aging populations. Those companies that develop particular orthopedic-specific regenerative solutions will be well placed to claim the lion's share of this emerging market since the industry is going progressively towards less-invasive and regenerative options.
- Collaborative Research Fuels Regenerative Medicine Advancements: Innovations in regenerative medicine are driven by cooperation among research institutions, hospitals, and biotech firms. In North America, 62% of the current clinical trials share multi-institutional collaboration, depicting the competitive advantage of resources and expertise. Therefore, there is growth opportunity as cooperative ventures drive the speed of discovery and therapeutic validation. Emerging collaborative frameworks have also been seen in geographies such as Europe and Asia-Pacific, which have led to breakthroughs in regenerative treatments.
Regenerative Medicine Market Regional Insights
The regional trends and factors influencing the Regenerative Medicine Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Regenerative Medicine Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Regenerative Medicine Market
Regenerative Medicine Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ XX million |
| Market Size by 2031 | US$ XX Million |
| Global CAGR (2025 - 2031) | 15.4% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Regenerative Medicine Market Players Density: Understanding Its Impact on Business Dynamics
The Regenerative Medicine Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Regenerative Medicine Market are:
- Integra LifeSciences Corporation
- Gilead Sciences
- Novartis
- Vericel Corporation
- Wright Medical
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Regenerative Medicine Market top key players overview
Key Selling Points
- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the Regenerative Medicine Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the Regenerative Medicine Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset


- Energy Recovery Ventilator Market
- Environmental Consulting Service Market
- Medical Devices Market
- Digital Pathology Market
- Nuclear Waste Management System Market
- Arterial Blood Gas Kits Market
- Excimer & Femtosecond Ophthalmic Lasers Market
- Hydrocephalus Shunts Market
- Sweet Potato Market
- Data Center Cooling Market

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Type, Application, and Geography

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
Some of the customization options available based on request are additional 3-5 company profiles and country-specific analysis of 3-5 countries of your choice. Customizations are to be requested/discussed before making final order confirmation, as our team would review the same and check the feasibility.
The report can be delivered in PDF/PPT format; we can also share excel dataset based on the request.
Key companies in this market are: Integra LifeSciences Corporation, Gilead Sciences, Novartis, Vericel Corporation, Wright Medical MiMedx, Osiris Therapeutics, Stryker Corporation, Spark Therapeutics
The Regenerative Medicine Market is expected to register a CAGR of 15.4% from 2023-2031.
Key future trends in this market are - Increase in stem cell research, Adoption of 3D bioprinting, Advances in tissue engineering
The major factors impacting the Regenerative Medicine Market are: Growing Focus on Stem Cell Research, Rising Prevalence of Chronic Diseases, and Advancements in Gene Therapy
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies
1. Integra LifeSciences Corporation
2. Gilead Sciences
3. Novartis
4. Vericel Corporation
5. Wright Medical
6. MiMedx
7. Osiris Therapeutics
8. Stryker Corporation
9. Spark Therapeutics
10. Medtronic
11. 3M
12. Allergan Plc
13. Aspect Biosystems
14. Bluebird Bio
15. Amgen, Inc.
16. Smith and Nephew Plc
17. Corestem, Inc.
18. Organogenesis
19. Kite Pharma
20. Spark Therapeutics
 Get Free Sample For
Get Free Sample For