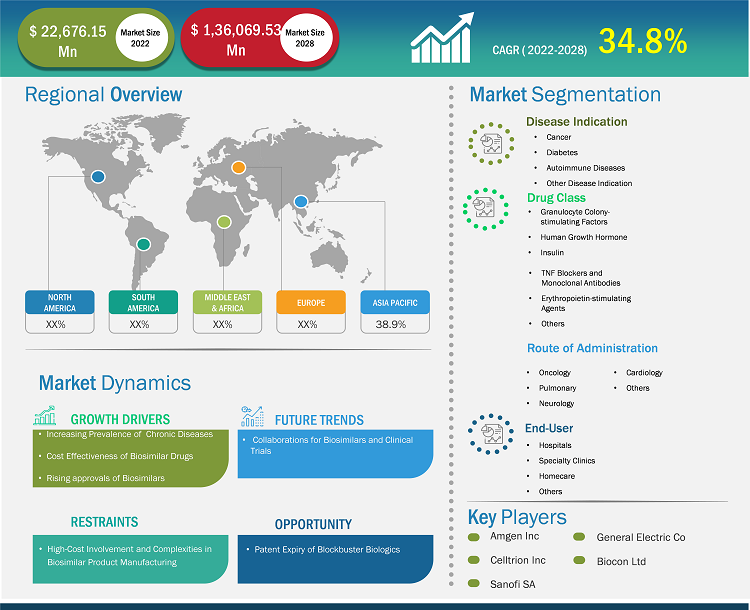
[Research Report] The biosimilars market size is expected to grow from US$ 18,435.89 million in 2021 to US$ 1,36,069.53 million in 2028; it is estimated to register a CAGR of 34.8% from 2022 to 2028.
Analyst Perspective
The major factors driving the growth of the biosimilars market are the growing incidence of chronic diseases, such as cancers, along. The growing burden of cancer and increasing deaths due to it creates the need for affordable treatment and thus boosts the growth of the biosimilars market. The key market players also anticipated market growth over the forecast period through various strategic activities, such as product launches, mergers, and acquisitions. An increase in the prevalence of autoimmune diseases such as ankylosing spondylitis and rheumatoid arthritis drives the growth of the Biosimilars Market Size. For instance, according to a paper published in “Scandinavian Journal of Rheumatology,” in 2020, titled ‘Prevalence of ankylosing spondylitis in Spain,’ about 7.3% population showed positive screening for ankylosing spondylitis. Biosimilars, such as infliximab-axxq (Avsola), infliximab-qbtx (Ixifi), infliximab-dyyb (Inflectra), and infliximab-abda (Renflexis) are used for the treatment of chronic pain in arthritis.
Market Overview
A biosimilar is a biological medicine highly similar to another already approved biological medicine (the 'reference medicine'). Biosimilars are approved according to the same standards of pharmaceutical quality, safety, and efficacy that apply to all biological medicines.Biosimilars are safe and effective treatment options for many illnesses, such as chronic skin and bowel diseases (like psoriasis, irritable bowel syndrome, Crohn’s disease, and colitis), arthritis, kidney conditions, and cancer. Biosimilars increase access to lifesaving medications at potentially lower costs. The primary drivers of the biosimilars market are increasing incidence of chronic diseases.
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
Biosimilars Market: Strategic Insights
Market Size Value in US$ 18,435.89 million in 2021 Market Size Value by US$ 1,36,069.53 million in 2028 Growth rate CAGR of 34.8% from 2022 to 2028 Forecast Period 2022-2028 Base Year 2021

Mrinal
Have a question?
Mrinal will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
Biosimilars Market: Strategic Insights

| Market Size Value in | US$ 18,435.89 million in 2021 |
| Market Size Value by | US$ 1,36,069.53 million in 2028 |
| Growth rate | CAGR of 34.8% from 2022 to 2028 |
| Forecast Period | 2022-2028 |
| Base Year | 2021 |

Mrinal
Have a question?
Mrinal will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Market Driver
Rising Approvals of Biosimilars to Drive Global Biosimilars Market Growth
The Food and Drug Administration (FDA) approves biosimilar products and provides the scientific and regulatory advice needed to bring safe and effective biosimilars to market. The approval of biosimilar products can improve patient care by increasing the number of medication options at potentially lower costs.
A few recent approvals of biosimilar products are mentioned in the following table.
Biosimilars Name | Approval Date | Reference Product |
Alymsys (bevacizumab-maly) | April 2022 | Avastin (bevacizumab) |
Cimerli (ranibizumab-eqrn) | August 2022 | Lucentis (ranibizumab) |
Fylnetra (pegfilgrastim-pbbk) | May 2022 | Neulasta (pegfilgrastim) |
Stimufend (pegfilgrastim-fpgk) | September 2022 | Neulasta (pegfilgrastim) |
Vegzelma (bevacizumab-adcd) | September 2022 | Avastin (bevacizumab) |
Idacio (adalimumab-aacf) | December 2022 | Humira (adalimumab) |
Byooviz (ranibizumab-nuna) | September 2021 | Lucentis (ranibizumab) |
Rezvoglar (insulin glargine-aglr) | December 2021 | Lantus (insulin glargine) |
Semglee (Insulin glargine-yfgn) | July 2021 | Lantus (Insulin glargine) |
Yusimry (adalimumab-aqvh) | December 2021 | Humira (adalimumab) |
Hulio (adalimumab-fkjp) | July 2020 | Humira (adalimumab) |
Riabni (rituximab-arrx) | December 2020 | Rituxan (rituximab) |
Nyvepria (pegfilgrastim-apgf) | June 2020 | Neulasta (pegfilgrastim) |
Thus, the rising approvals of biosimilars are propelling the biosimilars market growth.
Segmental Analysis
Based on disease indication, the biosimilars market is segmented into cancer, diabetes, autoimmune disease, and other disease indication. The cancer segment held the largest market share in 2021 and autoimmune disease is anticipated to register the highest CAGR of 36.1% during the forecast period (2022–2028). Based on drug class, the biosimilars market is segmented as granulocyte colony-stimulating factors, human growth hormone, insulin, TNF blockers and monoclonal antibodies, and others (osteoporosis, etc). The granulocyte colony-stimulating factors drug class segment held the largest share of the market in 2021. Moreover, the other drug class segment is expected to grow at the highest CAGR during the forecast period. Based on application, the global biosimilars market is divided into intravenous, subcutaneous, and other applications. The intravenous segment held the largest share of the market in 2021 and is expected to grow at the highest CAGR during the forecast period. The biosimilars market, by end-user, is segmented into hospitals, specialty clinics, homecare, and other end users. The hospitals segment held the largest share of the market in 2021 and homecare segment is anticipated to register the highest CAGR of 36.6% in the market during the forecast period (2022–2028).
Regional Analysis
The North America biosimilars market was valued at US$ 5,479.84 million in 2021 and is projected to reach US$ 47,746.80 million by 2028; it is expected to grow at a CAGR of 37.3% during the forecast period. The North America biosimilars market is segmented into the US, Canada, and Mexico. The US held the largest share of the North American biosimilars market in 2019. increasing incidence of diabetes, infertility as well as rising product development in biosimilars market. According to NIH Autoimmune Diseases Coordinating Committee, in 2019, more than 24 million Americans suffer from autoimmune diseases. Eight million people have auto-antibodies, blood molecules that indicate a person's risk of developing autoimmune diseases. For unknown reasons, autoimmune diseases are affecting more people. According to the Clinical Research Branch at the National Institute of Environmental Health Sciences (NIEHS), in 2020, there is a significant increase in the prevalence of antinuclear antibodies (ANA), the most common biomarker of autoimmunity in the US. The study is the first to evaluate ANA changes in a representative sampling of the US population over time. It includes males, non-Hispanic whites, adults over 50, and adolescents. In the US, biosimilars are used to treat patients with cancers, kidney diseases, diabetes, and other autoimmune diseases such as rheumatoid arthritis and Crohn's disease. According to Cardinal Health, a total of 33 biosimilars have been approved by the FDA in the US and 21 are commercially available. Of the 21 biosimilars on the market, 17 are used for treatments associated with cancers, three are used to treat autoimmune conditions and one is used to treat diabetes.
Biologics are the most expensive medicines in the US with costs totaling tens of thousands of dollars each year per patient. Biosimilars are expected to be priced 15% to 30% lower than their reference product. In 2020 alone, biosimilars saved US$ 7.9 billion, with savings expected to grow significantly in the next few years as more biosimilars enter the market. According to Cardinal Health, it is expected that biosimilars are expected to reduce US drug expenditure by US$ 133 billion by 2025. Thus, in the US, biosimilars have immense potential, for lowering the costs of biologic medicine and making care more accessible to patients, and for creating new innovations and scientific breakthroughs, thereby driving the biosimilars market growth in this region
Key Player Analysis
The biosimilars market analysis consists of players, such as Amgen Inc, Celltrion Inc, Sanofi SA, Biocon Ltd, Pfizer Inc, Samsung Bioepis Co Ltd, Coherus BioSciences Inc, Eli Lilly and Co, Sandoz AG, Teva Pharmaceutical Industries Ltd, and Dr. Reddy's Laboratories Ltd. Among the players in the biosimilars market, Pfizer Inc. and Novartis, Inc are the top two players owing to the diversified product portfolio offered.
Biosimilars Market Report Scope
Recent Developments
Inorganic and organic strategies such as mergers and acquisitions, product launches are highly adopted by companies in the biosimilars market. A few recent key market developments are listed below:
- In January 2022, Biocon Biologics a subsidiary of Biocon Ltd. Completed Acquisition of Viatris’ Global Biosimilars Business. The acquisition provides Biocon Biologics with direct commercial capabilities and supporting infrastructure in the advanced markets and several emerging markets, bringing it closer to patients, customers, and payors. With this acquisition Biocon Biologics emerges as a world leading biosimilars player with eight commercialized products.
- In October 2022, Biocon Biologics Out-Licenses Two Biosimilar Assets to Yoshindo for Commercialization in Japan. Under the terms of this deal, Yoshindo gets exclusive commercialization rights in Japan for bUstekinumab and bDenosumab developed and manufactured by Biocon Biologics, for an addressable market opportunity of US$ 700 million.
- In December 2022, Celltrion USA announced submission of the Biologics License Application (BLA) of novel subcutaneous formulation of CT-P13 to FDA. A subcutaneous formulation has the potential to enhance treatment options for the use of the infliximab drug by providing high consistency in drug exposure and a convenient method of administration.
- In September 2022, Celltrion USA received U.S. FDA approval for its oncology biosimilar Vegzelma for the treatment of six types of cancer such as metastatic colorectal cancer; recurrent or metastatic non-squamous non-small cell lung cancer (nsNSCLC); recurrent glioblastoma; metastatic renal cell carcinoma; persistent, recurrent, or metastatic cervical cancer; and epithelial ovarian, fallopian tube, or primary peritoneal cancer. Vegzelma is Celltrion’s third oncology biosimilar to receive approval from the U.S. FDA.
- In May 2022, Biocon Biologics and Viatris Launch Abevmy. Biocon Biologics Ltd., a subsidiary of Biocon Ltd., and Viatris Inc. announced that Abevmy (bBevacizumab) is available in Canada. Abevmy, co-developed by Biocon Biologics and Viatris, is a biosimilar to Roche’s Avastin (Bevacizumab) and has been approved by Health Canada across four oncology indications.

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Disease Indication, Drug Class, Route of Administration, and End User

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
France, Germany, Italy, Spain, United Kingdom
Frequently Asked Questions
The Asia Pacific is expected to be the fastest-growing region in the Biosimilars market over the forecast period due to the increasing prevalence of chronic diseases and the cost-effectiveness of biosimilars drugs.
The Biosimilars market is estimated to be valued at US$ 22,676.15 million in 2022.
The Biosimilars market is expected to be valued at US$ 1,36,069.53 million in 2028.
Biosimilars are safe and effective treatment options for many illnesses such as chronic skin and bowel diseases (like psoriasis, irritable bowel syndrome, Crohn’s disease and colitis), arthritis, kidney conditions, and cancer. A biosimilar product is a biologic product that is approved based on demonstrating that it is highly similar to an FDAâ€approved biologic product, known as a reference product, and has no clinically meaningful differences in terms of safety and effectiveness from the reference product.
The CAGR value of the biosimilars market during the forecasted period of 2022-2028 is 34.8%.
The Biosimilars market majorly consists of the players, such as Biocon Ltd, Sanofi-Aventis, Celltrion Inc., Amgen Inc., Pfizer Inc., Samsung Bioepis, Sanofi SA, Coherus BioSciences Inc, Dr. Reddy’s Laboratories Ltd, Eli Lilly and Co, Sandoz AG, and Teva Pharmaceutical Industries Ltd.
The factors that are driving the growth of the biosimilars market are the increasing aging population, changing social behavior, and the rising adoption of a sedentary lifestyle by people with accelerating urbanization boost the prevalence of obesity and various chronic diseases, such as diabetes. Also,twin studies have long established that genes can cause chronic conditions such as cardiovascular disease (CVDs), diabetes, obesity, Alzheimer's disease (AD), and depression. These are some of the major factors contributing to the growth of the biosimilars industry.
The insulin segment held the largest share of the market in 2022. Also, the same segment is estimated to register the highest CAGR in the market during the forecast period.
1. Introduction 30
1.1 Scope of the Study. 30
1.2 The Insight Partners Research Report Guidance. 30
1.3 Market Segmentation. 31
1.3.1 Global Biosimilars Market – by Disease Indication. 33
1.3.2 Global Biosimilars Market – by Drug class. 33
1.3.3 Global Biosimilars Market – by Route of Administration. 33
1.3.4 Global Biosimilars Market – by End User 33
1.3.5 Global Biosimilars Market – by Geography. 33
2. Biosimilars Market – Key Takeaways 35
3. Research Methodology 42
3.1 Coverage. 43
3.2 Secondary Research. 43
3.3 Primary Research. 43
4. Biosimilars Market – Market Landscape 45
4.1 Overview.. 45
4.2 PEST Analysis. 45
4.2.1 North America PEST Analysis. 45
4.2.2 Europe PEST Analysis. 46
4.2.3 Asia Pacific PEST Analysis. 46
4.2.4 South & Central America PEST Analysis. 47
4.2.5 Middle East & Africa PEST Analysis. 47
4.3 Expert’s Opinion. 48
5. Biosimilars Market – Key Market Dynamics 49
5.1 Market Drivers. 49
5.1.1 Increasing Prevalence of Chronic Diseases. 49
5.1.2 Cost Effectiveness of Biosimilar Drugs. 49
5.1.3 Rising Approvals of Biosimilars. 50
5.2 Market Restraints. 51
5.2.1 High-Cost Involvement and Complexities in Biosimilar Product Manufacturing. 51
5.3 Market Opportunities. 52
5.3.1 Patent Expiry of Blockbuster Biologics. 52
5.4 Future Trend. 53
5.4.1 Collaborations for Biosimilars and Clinical Trials. 53
5.5 Impact analysis. 54
6. Biosimilars Market – Global Analysis 55
6.1 Global Biosimilars Market Revenue Forecast and Analysis. 55
6.2 Global Biosimilars Market, by Geography – Forecast and Analysis. 56
6.3 Market Positioning of Key Players. 57
7. Global Biosimilars Market – Revenue and Forecast to 2028 – by Disease Indication 58
7.1 Overview.. 58
7.2 Biosimilars Market Revenue Share, by disease indication 2021 & 2028 (%) 58
7.3 Cancer 59
7.3.1 Overview.. 59
7.3.2 Cancer: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 59
7.4 Diabetes. 60
7.4.1 Overview.. 60
7.4.2 Diabetes: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 60
7.5 Autoimmune Diseases. 61
7.5.1 Overview.. 61
7.5.2 Autoimmune Diseases: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 61
7.5.3 Psoriasis: 62
7.5.3.1 Overview.. 62
7.5.3.2 Psoriasis: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 62
7.5.4 Arthritis: 63
7.5.4.1 Overview.. 63
7.5.4.2 Arthritis: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 63
7.5.5 Others: 64
7.5.5.1 Overview.. 64
7.5.5.2 Others: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 64
7.6 Others Disease Indications. 65
7.6.1 Overview.. 65
7.6.2 Others Disease Indications: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 65
8. Global Biosimilars Market Analysis and Forecasts to 2028 – by Route of Administration 66
8.1 Overview.. 66
8.2 Global Biosimilars Market, by Application 2021 & 2028 (%) 66
8.3 Intravenous. 67
8.3.1 Overview.. 67
8.3.2 Intravenous: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 67
8.4 Subcutaneous. 68
8.4.1 Overview.. 68
8.4.2 Subcutaneous: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 68
8.5 Others. 69
8.5.1 Overview.. 69
8.5.2 Others: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 69
9. Global Biosimilars Market – Revenue and Forecast to 2028 – by End User 70
9.1 Overview.. 70
9.2 Biosimilars Market Revenue Share, by End User 2021 & 2028 (%) 70
9.3 Hospitals. 71
9.3.1 Overview.. 71
9.3.2 Hospitals: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 71
9.4 Specialty Clinics. 72
9.4.1 Overview.. 72
9.4.2 Specialty Clinics: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 72
9.5 Homecare. 73
9.5.1 Overview.. 73
9.5.2 Homecare: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 73
9.6 Other 74
9.6.1 Overview.. 74
9.6.2 Other: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 74
10. Biosimilars Market – Revenue and Forecast to 2028 – Geographic Analysis 75
10.1 North America: Biosimilar Market Europe. 75
10.1.1 Overview.. 75
10.1.2 North America: Biosimilar Market - Revenue and Forecast to 2028 (US$ Million) 76
10.1.3 North America: Biosimilar Market, by Disease Indication, 2019–2028 (US$ Million) 76
10.1.3.1 North America: Biosimilar Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 77
10.1.4 North America: Biosimilar Market, by Drug Class, 2019–2028 (US$ Million) 77
10.1.5 North America: Biosimilar Market, by Route of Administration, 2019–2028 (US$ Million) 78
10.1.6 North America: Biosimilar Market, by End User, 2019–2028 (US$ Million) 78
10.1.7 North America: Biosimilar Market, by Country, 2021 & 2028 (%) 79
10.1.7.1 US: Biosimilar Market – Revenue and Forecast to 2028 (US$ Million) 79
10.1.7.1.1 Overview.. 79
10.1.7.1.2 US: Biosimilar Market - Revenue and Forecast to 2028 (US$ Million) 80
10.1.7.1.3 US: Biosimilar Market, by Disease Indication, 2019–2028 (US$ Million) 80
10.1.7.1.3.1 US: Biosimilar Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 81
10.1.7.1.4 US: Biosimilar Market, by Drug Class, 2019–2028 (US$ Million) 81
10.1.7.1.5 US: Biosimilar Market, by Route of Administration, 2019–2028 (US$ Million) 82
10.1.7.1.6 US: Biosimilar Market, by End User, 2019–2028 (US$ Million) 82
10.1.7.2 Canada: Biosimilar Market – Revenue and Forecast to 2028 (US$ Million) 83
10.1.7.2.1 Canada: Biosimilar Market - Revenue and Forecast to 2028 (US$ Million) 83
10.1.7.2.2 Canada: Biosimilar Market, by Disease Indication, 2019–2028 (US$ Million) 84
10.1.7.2.2.1 Canada: Biosimilar Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 84
10.1.7.2.3 Canada: Biosimilar Market, by Drug Class, 2019–2028 (US$ Million) 85
10.1.7.2.4 Canada: Biosimilar Market, by Route of Administration, 2019–2028 (US$ Million) 85
10.1.7.2.5 Canada: Biosimilar Market, by End User, 2019–2028 (US$ Million) 86
10.1.7.3 Mexico: Biosimilar Market – Revenue and Forecast to 2028 (US$ Million) 87
10.1.7.3.1 Mexico: Biosimilar Market - Revenue and Forecast to 2028 (US$ Million) 87
10.1.7.3.2 Mexico: Biosimilar Market, by Disease Indication, 2019–2028 (US$ Million) 88
10.1.7.3.2.1 Mexico: Biosimilar Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 88
10.1.7.3.3 Mexico: Biosimilar Market, by Drug Class, 2019–2028 (US$ Million) 88
10.1.7.3.4 Mexico: Biosimilar Market, by Route of Administration, 2019–2028 (US$ Million) 89
10.1.7.3.5 Mexico: Biosimilar Market, by End User, 2019–2028 (US$ Million) 89
10.2 Europe: Biosimilars Market 90
10.2.1 Overview.. 90
10.2.2 Europe: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 91
10.2.3 Europe: Biosimilars Market, by Disease Indication, 2019–2028 (US$ Million) 91
10.2.3.1 Europe: Biosimilars Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 92
10.2.4 Europe: Biosimilars Market, by Drug Class, 2019–2028 (US$ Million) 92
10.2.5 Europe: Biosimilars Market, by Route of Administration, 2019–2028 (US$ Million) 92
10.2.6 Europe: Biosimilars Market, by End User, 2019–2028 (US$ Million) 93
10.2.7 Europe: Biosimilars Market, by Country, 2021 & 2028 (%) 93
10.2.7.1 Germany: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 94
10.2.7.1.1 Overview.. 94
10.2.7.1.2 Germany: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 94
10.2.7.1.3 Germany: Biosimilars Market, by Disease Indication, 2019–2028 (US$ Million) 95
10.2.7.1.3.1 Germany: Biosimilars Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 95
10.2.7.1.4 Germany: Biosimilars Market, by Drug Class, 2019–2028 (US$ Million) 96
10.2.7.1.5 Germany: Biosimilars Market, by Route of Administration, 2019–2028 (US$ Million) 96
10.2.7.1.6 Germany Biosimilars Market, by End User, 2019–2028 (US$ Million) 96
10.2.7.2 UK: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 97
10.2.7.2.1 Overview.. 97
10.2.7.2.2 UK: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 97
10.2.7.2.3 UK: Biosimilars Market, by Disease Indication, 2019–2028 (US$ Million) 98
10.2.7.2.3.1 UK: Biosimilars Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 98
10.2.7.2.4 UK: Biosimilars Market, by Drug Class, 2019–2028 (US$ Million) 99
10.2.7.2.5 UK: Biosimilars Market, by Route of Administration, 2019–2028 (US$ Million) 99
10.2.7.2.6 UK: Biosimilars Market, by End User, 2019–2028 (US$ Million) 100
10.2.7.3 France: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 101
10.2.7.3.1 Overview.. 101
10.2.7.3.2 France: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 101
10.2.7.3.3 France: Biosimilars Market, by Disease Indication, 2019–2028 (US$ Million) 102
10.2.7.3.3.1 France: Biosimilars Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 102
10.2.7.3.4 France: Biosimilars Market, by Drug Class, 2019–2028 (US$ Million) 102
10.2.7.3.5 France: Biosimilars Market, by Route of Administration, 2019–2028 (US$ Million) 103
10.2.7.3.6 France: Biosimilars Market, by End User, 2019–2028 (US$ Million) 103
10.2.7.4 Italy: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 104
10.2.7.4.1 Overview.. 104
10.2.7.4.2 Italy: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 104
10.2.7.4.3 Italy: Biosimilars Market, by Disease Indication, 2019–2028 (US$ Million) 105
10.2.7.4.3.1 Italy: Biosimilars Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 105
10.2.7.4.4 Italy: Biosimilars Market, by Drug Class, 2019–2028 (US$ Million) 105
10.2.7.4.5 Italy: Biosimilars Market, by Route of Administration, 2019–2028 (US$ Million) 106
10.2.7.4.6 Italy: Biosimilars Market, by End User, 2019–2028 (US$ Million) 106
10.2.7.5 Spain: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 107
10.2.7.5.1 Overview.. 107
10.2.7.5.2 Spain: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 107
10.2.7.5.3 Spain: Biosimilars Market, by Disease Indication, 2019–2028 (US$ Million) 108
10.2.7.5.3.1 Spain: Biosimilars Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 109
10.2.7.5.4 Spain: Biosimilars Market, by Drug Class, 2019–2028 (US$ Million) 109
10.2.7.5.5 Spain: Biosimilars Market, by Route of Administration, 2019–2028 (US$ Million) 109
10.2.7.5.6 Spain: Biosimilars Market, by End User, 2019–2028 (US$ Million) 110
10.2.7.6 Rest of Europe: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 111
10.2.7.6.1 Overview.. 111
10.2.7.6.2 Rest of Europe: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 111
10.2.7.6.3 Rest of Europe: Biosimilars Market, by Disease Indication, 2019–2028 (US$ Million) 112
10.2.7.6.3.1 Rest of Europe: Biosimilars Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 112
10.2.7.6.4 Rest of Europe: Biosimilars Market, by Drug Class, 2019–2028 (US$ Million) 112
10.2.7.6.5 Rest of Europe: Biosimilars Market, by Route of Administration, 2019–2028 (US$ Million) 113
10.2.7.6.6 Rest of Europe: Biosimilars Market, by End User, 2019–2028 (US$ Million) 113
10.3 Asia Pacific: Biosimilars Market 114
10.3.1 Overview.. 114
10.3.2 Asia Pacific: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 115
10.3.3 Asia Pacific: Biosimilars Market, by Disease Indication, 2019–2028 (US$ Million) 115
10.3.3.1 Asia Pacific: Biosimilars Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 116
10.3.4 Asia Pacific: Biosimilars Market, by Drug Class, 2019–2028 (US$ Million) 116
10.3.5 Asia Pacific: Biosimilars Market, by Route of Administration, 2019–2028 (US$ Million) 117
10.3.6 Asia Pacific: Biosimilars Market, by End User, 2019–2028 (US$ Million) 117
10.3.7 Asia Pacific: Biosimilars Market, by Country, 2021 & 2028 (%) 117
10.3.7.1 China: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 118
10.3.7.1.1 Overview.. 118
10.3.7.1.2 China: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 118
10.3.7.1.3 China: Biosimilars Market, by Disease Indication, 2019–2028 (US$ Million) 119
10.3.7.1.3.1 China: Biosimilars Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 119
10.3.7.1.4 China: Biosimilars Market, by Drug Class, 2019–2028 (US$ Million) 120
10.3.7.1.5 China: Biosimilars Market, by Route of Administration, 2019–2028 (US$ Million) 120
10.3.7.1.6 China Biosimilars Market, by End User, 2019–2028 (US$ Million) 120
10.3.7.2 Japan: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 121
10.3.7.2.1 Overview.. 121
10.3.7.2.2 Japan: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 121
10.3.7.2.3 Japan: Biosimilars Market, by Disease Indication, 2019–2028 (US$ Million) 122
10.3.7.2.3.1 Japan: Biosimilars Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 122
10.3.7.2.4 Japan: Biosimilars Market, by Drug Class, 2019–2028 (US$ Million) 123
10.3.7.2.5 Japan: Biosimilars Market, by Route of Administration, 2019–2028 (US$ Million) 123
10.3.7.2.6 Japan: Biosimilars Market, by End User, 2019–2028 (US$ Million) 123
10.3.7.3 India: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 124
10.3.7.3.1 Overview.. 124
10.3.7.3.2 India: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 124
10.3.7.3.3 India: Biosimilars Market, by Disease Indication, 2019–2028 (US$ Million) 125
10.3.7.3.3.1 India: Biosimilars Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 125
10.3.7.3.4 India: Biosimilars Market, by Drug Class, 2019–2028 (US$ Million) 125
10.3.7.3.5 India: Biosimilars Market, by Route of Administration, 2019–2028 (US$ Million) 126
10.3.7.3.6 India: Biosimilars Market, by End User, 2019–2028 (US$ Million) 126
10.3.7.4 South Korea: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 127
10.3.7.4.1 Overview.. 127
10.3.7.4.2 South Korea: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 127
10.3.7.4.3 South Korea: Biosimilars Market, by Disease Indication, 2019–2028 (US$ Million) 127
10.3.7.4.3.1 South Korea: Biosimilars Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 128
10.3.7.4.4 South Korea: Biosimilars Market, by Drug Class, 2019–2028 (US$ Million) 128
10.3.7.4.5 South Korea: Biosimilars Market, by Route of Administration, 2019–2028 (US$ Million) 129
10.3.7.4.6 South Korea: Biosimilars Market, by End User, 2019–2028 (US$ Million) 129
10.3.7.5 Australia: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 130
10.3.7.5.1 Overview.. 130
10.3.7.5.2 Australia: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 130
10.3.7.5.3 Australia: Biosimilars Market, by Disease Indication, 2019–2028 (US$ Million) 130
10.3.7.5.3.1 Australia: Biosimilars Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 131
10.3.7.5.4 Australia: Biosimilars Market, by Drug Class, 2019–2028 (US$ Million) 131
10.3.7.5.5 Australia: Biosimilars Market, by Route of Administration, 2019–2028 (US$ Million) 132
10.3.7.5.6 Australia: Biosimilars Market, by End User, 2019–2028 (US$ Million) 132
10.3.7.6 Rest of Asia Pacific: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 133
10.3.7.6.1 Overview.. 133
10.3.7.6.2 Rest of Asia Pacific: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 133
10.3.7.6.3 Rest of Asia Pacific: Biosimilars Market, by Disease Indication, 2019–2028 (US$ Million) 134
10.3.7.6.3.1 Rest of Asia Pacific: Biosimilars Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 134
10.3.7.6.4 Rest of Asia Pacific: Biosimilars Market, by Drug Class, 2019–2028 (US$ Million) 135
10.3.7.6.5 Rest of Asia Pacific: Biosimilars Market, by Route of Administration, 2019–2028 (US$ Million) 135
10.3.7.6.6 Rest of Asia Pacific: Biosimilars Market, by End User, 2019–2028 (US$ Million) 135
10.4 Middle East and Africa: Biosimilars Market 137
10.4.1 Overview.. 137
10.4.2 Middle East and Africa: Biosimilar Market - Revenue and Forecast to 2028 (US$ Million) 138
10.4.3 Middle East and Africa: Biosimilar Market, by Disease Indication, 2019–2028 (US$ Million) 138
10.4.3.1 Middle East and Africa: Biosimilar Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 139
10.4.4 Middle East and Africa: Biosimilar Market, by Drug Class, 2019–2028 (US$ Million) 139
10.4.5 Middle East and Africa: Biosimilar Market, by Route of Administration, 2019–2028 (US$ Million) 139
10.4.6 Middle East and Africa: Biosimilar Market, by End User, 2019–2028 (US$ Million) 140
10.4.7 Middle East and Africa: Biosimilar Market, by Country, 2021 & 2028 (%) 140
10.4.7.1 Saudi Arabia: Biosimilar Market – Revenue and Forecast to 2028 (US$ Million) 141
10.4.7.1.1 Overview.. 141
10.4.7.1.2 Saudi Arabia: Biosimilar Market - Revenue and Forecast to 2028 (US$ Million) 141
10.4.7.1.3 Saudi Arabia: Biosimilar Market, by Disease Indication, 2019–2028 (US$ Million) 142
10.4.7.1.3.1 Saudi Arabia: Biosimilar Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 142
10.4.7.1.4 Saudi Arabia: Biosimilar Market, by Drug Class, 2019–2028 (US$ Million) 142
10.4.7.1.5 Saudi Arabia: Biosimilar Market, by Route of Administration, 2019–2028 (US$ Million) 143
10.4.7.1.6 Saudi Arabia: Biosimilar Market, by End User, 2019–2028 (US$ Million) 143
10.4.7.2 South Africa: Biosimilar Market – Revenue and Forecast to 2028 (US$ Million) 144
10.4.7.2.1 South Africa: Biosimilar Market - Revenue and Forecast to 2028 (US$ Million) 144
10.4.7.2.2 South Africa: Biosimilar Market, by Disease Indication, 2019–2028 (US$ Million) 145
10.4.7.2.2.1 South Africa: Biosimilar Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 145
10.4.7.2.3 South Africa: Biosimilar Market, by Drug Class, 2019–2028 (US$ Million) 145
10.4.7.2.4 South Africa: Biosimilar Market, by Route of Administration, 2019–2028 (US$ Million) 146
10.4.7.2.5 South Africa: Biosimilar Market, by End User, 2019–2028 (US$ Million) 146
10.4.7.3 UAE: Biosimilar Market – Revenue and Forecast to 2028 (US$ Million) 147
10.4.7.3.1 UAE: Biosimilar Market - Revenue and Forecast to 2028 (US$ Million) 147
10.4.7.3.2 UAE: Biosimilar Market, by Disease Indication, 2019–2028 (US$ Million) 148
10.4.7.3.2.1 UAE: Biosimilar Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 148
10.4.7.3.3 UAE: Biosimilar Market, by Drug Class, 2019–2028 (US$ Million) 148
10.4.7.3.4 UAE: Biosimilar Market, by Route of Administration, 2019–2028 (US$ Million) 149
10.4.7.3.5 UAE: Biosimilar Market, by End User, 2019–2028 (US$ Million) 149
10.4.7.4 Rest of Middle East and Africa: Biosimilar Market – Revenue and Forecast to 2028 (US$ Million) 150
10.4.7.4.1 Rest of Middle East and Africa: Biosimilar Market - Revenue and Forecast to 2028 (US$ Million) 150
10.4.7.4.2 Rest of Middle East and Africa: Biosimilar Market, by Disease Indication, 2019–2028 (US$ Million) 150
10.4.7.4.2.1 Rest of Middle East and Africa: Biosimilar Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 151
10.4.7.4.3 Rest of Middle East and Africa: Biosimilar Market, by Drug Class, 2019–2028 (US$ Million) 151
10.4.7.4.4 Rest of Middle East and Africa: Biosimilar Market, by Route of Administration, 2019–2028 (US$ Million) 152
10.4.7.4.5 Rest of Middle East and Africa: Biosimilar Market, by End User, 2019–2028 (US$ Million) 152
10.5 South & Central America Biosimilars Market 153
10.5.1 Overview.. 153
10.5.2 South & Central America: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 153
10.5.3 South & Central America: Biosimilars Market, by Disease Indication, 2019–2028 (US$ Million) 154
10.5.3.1 South & Central America: Biosimilars Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 154
10.5.4 South & Central America: Biosimilars Market, by Drug Class, 2019–2028 (US$ Million) 154
10.5.5 South & Central America: Biosimilars Market, by Route of Administration, 2019–2028 (US$ Million) 155
10.5.6 South & Central America: Biosimilars Market, by End User, 2019–2028 (US$ Million) 155
10.5.7 South & Central America: Biosimilars Market, by Country, 2021 & 2028 (%) 156
10.5.7.1 Brazil: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 156
10.5.7.1.1 Overview.. 156
10.5.7.1.2 Brazil: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 156
10.5.7.1.3 Brazil: Biosimilars Market, by Disease Indication, 2019–2028 (US$ Million) 157
10.5.7.1.3.1 Brazil: Biosimilars Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 157
10.5.7.1.4 Brazil: Biosimilars Market, by Drug Class, 2019–2028 (US$ Million) 157
10.5.7.1.5 Brazil: Biosimilars Market, by Route of Administration, 2019–2028 (US$ Million) 158
10.5.7.1.6 Brazil: Biosimilars Market, by End User, 2019–2028 (US$ Million) 158
10.5.7.2 Argentina: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 159
10.5.7.2.1 Overview.. 159
10.5.7.2.2 Argentina: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 159
10.5.7.2.3 Argentina: Biosimilars Market, by Disease Indication, 2019–2028 (US$ Million) 159
10.5.7.2.3.1 Argentina: Biosimilars Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 160
10.5.7.2.4 Argentina: Biosimilars Market, by Drug Class, 2019–2028 (US$ Million) 160
10.5.7.2.5 Argentina: Biosimilars Market, by Route of Administration, 2019–2028 (US$ Million) 161
10.5.7.2.6 Argentina: Biosimilars Market, by End User, 2019–2028 (US$ Million) 161
10.5.7.3 Rest of South & Central America: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 162
10.5.7.3.1 Overview.. 162
10.5.7.3.2 Rest of South & Central America: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 162
10.5.7.3.3 Rest of South & Central America: Biosimilars Market, by Disease Indication, 2019–2028 (US$ Million) 163
10.5.7.3.3.1 Rest of South & Central America: Biosimilars Market, by Autoimmune Diseases, 2019–2028 (US$ Million) 163
10.5.7.3.4 Rest of South & Central America: Biosimilars Market, by Drug Class, 2019–2028 (US$ Million) 164
10.5.7.3.5 Rest of South & Central America: Biosimilars Market, by Route of Administration, 2019–2028 (US$ Million) 164
10.5.7.3.6 Rest of South & Central America: Biosimilars Market, by End User, 2019–2028 (US$ Million) 165
11. Biosimilars Market – Industry Landscape 166
11.1 Overview.. 166
11.2 Growth Strategies in the Biosimilars Market 166
11.3 Inorganic Growth Strategies. 167
11.3.1 Overview.. 167
11.4 Organic Growth Strategies. 169
11.4.1 Overview.. 169
12. Company Profiles 172
12.1 Amgen Inc. 172
12.1.1 Key Facts. 172
12.1.2 Business Description. 172
12.1.3 Products and Services. 172
12.1.4 Financial Overview.. 173
12.1.5 SWOT Analysis. 175
12.1.6 Key Developments. 175
12.2 Celltrion Inc. 176
12.2.1 Key Facts. 176
12.2.2 Business Description. 176
12.2.3 Products and Services. 176
12.2.4 Financial Overview.. 176
12.2.5 SWOT Analysis. 178
12.2.6 Key Developments. 179
12.3 Sanofi SA. 180
12.3.1 Key Facts. 180
12.3.2 Business Description. 180
12.3.3 Products and Services. 181
12.3.4 Financial Overview.. 181
12.3.5 SWOT Analysis. 183
12.3.6 Key Developments. 183
12.4 Biocon Ltd. 184
12.4.1 Key Facts. 184
12.4.2 Business Description. 184
12.4.3 Products and Services. 184
12.4.4 Financial Overview.. 185
12.4.5 SWOT Analysis. 186
12.4.6 Key Developments. 187
12.5 Samsung Bioepis Co Ltd. 188
12.5.1 Key Facts. 188
12.5.2 Business Description. 188
12.5.3 Products and Services. 188
12.5.4 Financial Overview.. 188
12.5.5 SWOT Analysis. 189
12.5.6 Key Developments. 190
12.6 Coherus BioSciences Inc. 191
12.6.1 Key Facts. 191
12.6.2 Business Description. 191
12.6.3 Products and Services. 191
12.6.4 Financial Overview.. 191
12.6.5 SWOT Analysis. 193
12.6.6 Key Developments. 194
12.7 Eli Lilly and Co. 195
12.7.1 Key Facts. 195
12.7.2 Business Description. 195
12.7.3 Products and Services. 196
12.7.4 Financial Overview.. 196
12.7.5 SWOT Analysis. 198
12.7.6 Key Developments. 198
12.8 Sandoz AG.. 199
12.8.1 Key Facts. 199
12.8.2 Business Description. 199
12.8.3 Products and Services. 199
12.8.4 Financial Overview.. 199
12.8.5 SWOT Analysis. 200
12.8.6 Key Developments. 201
12.9 Teva Pharmaceutical Industries Ltd. 202
12.9.1 Key Facts. 202
12.9.2 Business Description. 202
12.9.3 Products and Services. 203
12.9.4 Financial Overview.. 203
12.9.5 SWOT Analysis. 205
12.9.6 Key Developments. 205
12.10 Pfizer Inc. 206
12.10.1 Key Facts. 206
12.10.2 Business Description. 206
12.10.3 Products and Services. 207
12.10.4 Financial Overview.. 207
12.10.5 SWOT Analysis. 209
12.10.6 Key Developments. 209
12.11 Dr. Reddy's Laboratories Ltd. 210
12.11.1 Key Facts. 210
12.11.2 Business Description. 210
12.11.3 Products and Services. 210
12.11.4 Financial Overview.. 211
12.11.5 SWOT Analysis. 212
12.11.6 Key Developments. 212
13. Appendix 214
13.1 About The Insight Partners. 214
13.2 Glossary of Terms. 214
LIST OF TABLES
Table 1. Comparison Between Different Drug Developments. 51
Table 2. North America Biosimilar Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 76
Table 3. North America Biosimilar Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 77
Table 4. North America Biosimilar Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 77
Table 5. North America Biosimilar Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 78
Table 6. North America Biosimilar Market, by End User – Revenue and Forecast to 2028 (US$ Million) 78
Table 7. US Biosimilar Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 80
Table 8. US Biosimilar Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 81
Table 9. US Biosimilar Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 81
Table 10. US Biosimilar Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 82
Table 11. US Biosimilar Market, by End User – Revenue and Forecast to 2028 (US$ Million) 82
Table 12. Canada Biosimilar Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 84
Table 13. Canada Biosimilar Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 84
Table 14. Canada Biosimilar Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 85
Table 15. Canada Biosimilar Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 85
Table 16. Canada Biosimilar Market, by End User – Revenue and Forecast to 2028 (US$ Million) 86
Table 17. Mexico Biosimilar Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 88
Table 18. Mexico Biosimilar Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 88
Table 19. Mexico Biosimilar Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 88
Table 20. Mexico Biosimilar Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 89
Table 21. Mexico Biosimilar Market, by End User – Revenue and Forecast to 2028 (US$ Million) 89
Table 22. Europe Biosimilars Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 91
Table 23. Europe Biosimilars Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 92
Table 24. Europe Biosimilars Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 92
Table 25. Europe Biosimilars Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 92
Table 26. Europe Biosimilars Market, by End User – Revenue and Forecast to 2028 (US$ Million) 93
Table 27. Germany Biosimilars Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 95
Table 28. Germany Biosimilars Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 95
Table 29. Germany Biosimilars Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 96
Table 30. Germany Biosimilars Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 96
Table 31. Germany Biosimilars Market, by End User – Revenue and Forecast to 2028 (US$ Million) 96
Table 32. UK Biosimilars Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 98
Table 33. UK Biosimilars Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 98
Table 34. UK Biosimilars Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 99
Table 35. UK Biosimilars Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 99
Table 36. UK Biosimilars Market, by End User – Revenue and Forecast to 2028 (US$ Million) 100
Table 37. France Biosimilars Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 102
Table 38. France Biosimilars Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 102
Table 39. France Biosimilars Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 102
Table 40. France Biosimilars Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 103
Table 41. France Biosimilars Market, by End User – Revenue and Forecast to 2028 (US$ Million) 103
Table 42. Italy Biosimilars Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 105
Table 43. Italy Biosimilars Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 105
Table 44. Italy Biosimilars Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 105
Table 45. Italy Biosimilars Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 106
Table 46. Italy Biosimilars Market, by End User – Revenue and Forecast to 2028 (US$ Million) 106
Table 47. Spain Biosimilars Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 108
Table 48. Spain Biosimilars Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 109
Table 49. Spain Biosimilars Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 109
Table 50. Spain Biosimilars Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 109
Table 51. Spain Biosimilars Market, by End User – Revenue and Forecast to 2028 (US$ Million) 110
Table 52. Rest of Europe Biosimilars Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 112
Table 53. Rest of Europe Biosimilars Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 112
Table 54. Rest of Europe Biosimilars Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 112
Table 55. Rest of Europe Biosimilars Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 113
Table 56. Rest of Europe Biosimilars Market, by End User – Revenue and Forecast to 2028 (US$ Million) 113
Table 57. Asia Pacific Biosimilars Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 115
Table 58. Asia Pacific Biosimilars Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 116
Table 59. Asia Pacific Biosimilars Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 116
Table 60. Asia Pacific Biosimilars Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 117
Table 61. Asia Pacific Biosimilars Market, by End User – Revenue and Forecast to 2028 (US$ Million) 117
Table 62. China Biosimilars Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 119
Table 63. China Biosimilars Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 119
Table 64. China Biosimilars Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 120
Table 65. China Biosimilars Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 120
Table 66. China Biosimilars Market, by End User – Revenue and Forecast to 2028 (US$ Million) 120
Table 67. Japan Biosimilars Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 122
Table 68. Japan Biosimilars Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 122
Table 69. Japan Biosimilars Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 123
Table 70. Japan Biosimilars Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 123
Table 71. Japan Biosimilars Market, by End User – Revenue and Forecast to 2028 (US$ Million) 123
Table 72. India Biosimilars Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 125
Table 73. India Biosimilars Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 125
Table 74. India Biosimilars Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 125
Table 75. India Biosimilars Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 126
Table 76. India Biosimilars Market, by End User – Revenue and Forecast to 2028 (US$ Million) 126
Table 77. South Korea Biosimilars Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 127
Table 78. South Korea Biosimilars Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 128
Table 79. South Korea Biosimilars Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 128
Table 80. South Korea Biosimilars Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 129
Table 81. South Korea Biosimilars Market, by End User – Revenue and Forecast to 2028 (US$ Million) 129
Table 82. Australia Biosimilars Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 130
Table 83. Australia Biosimilars Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 131
Table 84. Australia Biosimilars Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 131
Table 85. Australia Biosimilars Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 132
Table 86. Australia Biosimilars Market, by End User – Revenue and Forecast to 2028 (US$ Million) 132
Table 87. Rest of Asia Pacific Biosimilars Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 134
Table 88. Rest of Asia Pacific Biosimilars Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 134
Table 89. Rest of Asia Pacific Biosimilars Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 135
Table 90. Rest of Asia Pacific Biosimilars Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 135
Table 91. Rest of Asia Pacific Biosimilars Market, by End User – Revenue and Forecast to 2028 (US$ Million) 135
Table 92. Middle East and Africa Biosimilar Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 138
Table 93. Middle East and Africa Biosimilar Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 139
Table 94. Middle East and Africa Biosimilar Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 139
Table 95. Middle East and Africa Biosimilar Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 139
Table 96. Middle East and Africa Biosimilar Market, by End User – Revenue and Forecast to 2028 (US$ Million) 140
Table 97. Saudi Arabia Biosimilar Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 142
Table 98. Saudi Arabia Biosimilar Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 142
Table 99. Saudi Arabia Biosimilar Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 142
Table 100. Saudi Arabia Biosimilar Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 143
Table 101. Saudi Arabia Biosimilar Market, by End User – Revenue and Forecast to 2028 (US$ Million) 143
Table 102. South Africa Biosimilar Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 145
Table 103. South Africa Biosimilar Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 145
Table 104. South Africa Biosimilar Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 145
Table 105. South Africa Biosimilar Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 146
Table 106. South Africa Biosimilar Market, by End User – Revenue and Forecast to 2028 (US$ Million) 146
Table 107. UAE Biosimilar Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 148
Table 108. UAE Biosimilar Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 148
Table 109. UAE Biosimilar Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 148
Table 110. UAE Biosimilar Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 149
Table 111. UAE Biosimilar Market, by End User – Revenue and Forecast to 2028 (US$ Million) 149
Table 112. Rest of Middle East and Africa Biosimilar Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 150
Table 113. Rest of Middle East and Africa Biosimilar Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 151
Table 114. Rest of Middle East and Africa Biosimilar Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 151
Table 115. Rest of Middle East and Africa Biosimilar Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 152
Table 116. Rest of Middle East and Africa Biosimilar Market, by End User – Revenue and Forecast to 2028 (US$ Million) 152
Table 117. South & Central America Biosimilars Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 154
Table 118. South & Central America Biosimilars Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 154
Table 119. South & Central America Biosimilars Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 154
Table 120. South & Central America Biosimilars Market, by Route of Administration – Revenue and Forecast to 2028 (US$ Million) 155
Table 121. South & Central America Biosimilars Market, by End User – Revenue and Forecast to 2028 (US$ Million) 155
Table 122. Brazil Biosimilars Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 157
Table 123. Brazil Biosimilars Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 157
Table 124. Brazil Biosimilars Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 157
Table 125. Brazil Biosimilars Market, by Route of Administration– Revenue and Forecast to 2028 (US$ Million) 158
Table 126. Brazil Biosimilars Market, by End User – Revenue and Forecast to 2028 (US$ Million) 158
Table 127. Argentina Biosimilars Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 159
Table 128. Argentina Biosimilars Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 160
Table 129. Argentina Biosimilars Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 160
Table 130. Argentina Biosimilars Market, by Route of Administration– Revenue and Forecast to 2028 (US$ Million) 161
Table 131. Argentina Biosimilars Market, by End User – Revenue and Forecast to 2028 (US$ Million) 161
Table 132. Rest of South & Central America Biosimilars Market, by Disease Indication – Revenue and Forecast to 2028 (US$ Million) 163
Table 133. Rest of South & Central America Biosimilars Market, by Autoimmune Diseases – Revenue and Forecast to 2028 (US$ Million) 163
Table 134. Rest of South & Central America Biosimilars Market, by Drug Class – Revenue and Forecast to 2028 (US$ Million) 164
Table 135. Rest of South & Central America Biosimilars Market, by Route of Administration– Revenue and Forecast to 2028 (US$ Million) 164
Table 136. Rest of South & Central America Biosimilars Market, by End User – Revenue and Forecast to 2028 (US$ Million) 165
Table 137. Recent Inorganic Growth Strategies in the Biosimilars Market 167
Table 138. Recent Organic Growth Strategies in the Biosimilars Market 169
Table 139. Glossary of Terms. 214
LIST OF FIGURES
Figure 1. Biosimilars Market Segmentation. 31
Figure 2. Biosimilars Market, by Region. 32
Figure 3. Global Biosimilars Market Overview. 35
Figure 4. Cancer Segment Held Largest Share of Type Segment in Biosimilars Market 36
Figure 5. Asia Pacific Expected to Show Remarkable Growth During Forecast Period. 37
Figure 6. Biosimilars Market, by Geography (US$ Million) 38
Figure 7. Global Biosimilars Market – Leading Country Markets (US$ Million) 39
Figure 8. Global Biosimilars Market – Industry Landscape. 40
Figure 9. North America: PEST Analysis. 45
Figure 10. Europe: PEST Analysis. 46
Figure 11. Asia Pacific: PEST Analysis. 46
Figure 12. South & Central America: PEST Analysis. 47
Figure 13. Middle East & Africa: PEST Analysis. 47
Figure 14. Experts’ Opinion. 48
Figure 15. Biosimilars Market Impact Analysis of Drivers and Restraints. 54
Figure 16. Global Biosimilars Market – Revenue Forecast and Analysis – 2020–2028. 55
Figure 17. Global Biosimilars Market, by Geography – Forecast and Analysis (2021–2028) 56
Figure 18. Market Positioning of Key Players in Biosimilars Market 57
Figure 19. Biosimilars Market Revenue Share, by disease indication 2021 & 2028 (%) 58
Figure 20. Cancer: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 59
Figure 21. Diabetes: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 60
Figure 22. Autoimmune Diseases: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 61
Figure 23. Psoriasis: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 62
Figure 24. Arthritis: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 63
Figure 25. Others: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 64
Figure 26. Others Disease Indications: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 65
Figure 27. Global Biosimilars Market, by Application 2021 & 2028 (%) 66
Figure 28. Intravenous: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 67
Figure 29. Subcutaneous: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 68
Figure 30. Others: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 69
Figure 31. Biosimilars Market Revenue Share, by End User 2021 & 2028 (%) 70
Figure 32. Hospitals: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 71
Figure 33. Specialty Clinics: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 72
Figure 34. Homecare: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 73
Figure 35. Other: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 74
Figure 36. North America: Biosimilar Market, by Key Country – Revenue (2022) (US$ Million) 75
Figure 37. North America Biosimilar Market Revenue and Forecast to 2028 (USD Million) 76
Figure 38. US Biosimilar Market Revenue and Forecast to 2028 (USD Million) 80
Figure 39. Canada Biosimilar Market Revenue and Forecast to 2028 (USD Million) 83
Figure 40. Mexico Biosimilar Market Revenue and Forecast to 2028 (USD Million) 87
Figure 41. Europe: Biosimilars Market, by Key Country – Revenue (2021) (US$ Million) 90
Figure 42. Europe Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 91
Figure 43. Germany: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 94
Figure 44. UK: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 97
Figure 45. France: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 101
Figure 46. Italy: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 104
Figure 47. Spain: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 108
Figure 48. Rest of Europe: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 111
Figure 49. Asia Pacific: Biosimilars Market, by Key Country – Revenue (2021) (US$ Million) 114
Figure 50. Asia Pacific Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 115
Figure 51. China: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 118
Figure 52. Japan: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 121
Figure 53. India: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 124
Figure 54. South Korea: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 127
Figure 55. Australia: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 130
Figure 56. Rest of Asia Pacific: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 133
Figure 57. Middle East and Africa: Biosimilar Market, by Key Country – Revenue (2022) (US$ Million) 137
Figure 58. Middle East and Africa Biosimilar Market Revenue and Forecast to 2028 (USD Million) 138
Figure 59. Saudi Arabia Biosimilar Market Revenue and Forecast to 2028 (USD Million) 141
Figure 60. South Africa Biosimilar Market Revenue and Forecast to 2028 (USD Million) 144
Figure 61. UAE Biosimilar Market Revenue and Forecast to 2028 (USD Million) 147
Figure 62. Rest of Middle East and Africa Biosimilar Market Revenue and Forecast to 2028 (USD Million) 150
Figure 63. South & Central America: Biosimilars Market, by Key Country – Revenue (2021) (US$ Million) 153
Figure 64. South & Central America Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 153
Figure 65. South & Central America: Biosimilars Market, by Country, 2021 & 2028 (%) 156
Figure 66. Brazil: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 156
Figure 67. Argentina: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 159
Figure 68. Rest of South & Central America: Biosimilars Market – Revenue and Forecast to 2028 (US$ Million) 162
Figure 69. Growth Strategies in the Biosimilars Market 166
The List of Companies - Biosimilar Market
- Biocon Ltd
- Sanofi-Aventis
- Celltrion Inc.
- Amgen Inc.
- Pfizer Inc.
- Samsung Bioepis
- Sanofi SA
- Coherus BioSciences Inc
- Dr. Reddy’s Laboratories Ltd
- Eli Lilly and Co
- Sandoz AG
- Teva Pharmaceutical Industries Ltd.
The Insight Partners performs research in 4 major stages: Data Collection & Secondary Research, Primary Research, Data Analysis and Data Triangulation & Final Review.
- Data Collection and Secondary Research:
As a market research and consulting firm operating from a decade, we have published many reports and advised several clients across the globe. First step for any study will start with an assessment of currently available data and insights from existing reports. Further, historical and current market information is collected from Investor Presentations, Annual Reports, SEC Filings, etc., and other information related to company’s performance and market positioning are gathered from Paid Databases (Factiva, Hoovers, and Reuters) and various other publications available in public domain.
Several associations trade associates, technical forums, institutes, societies and organizations are accessed to gain technical as well as market related insights through their publications such as research papers, blogs and press releases related to the studies are referred to get cues about the market. Further, white papers, journals, magazines, and other news articles published in the last 3 years are scrutinized and analyzed to understand the current market trends.
- Primary Research:
The primarily interview analysis comprise of data obtained from industry participants interview and answers to survey questions gathered by in-house primary team.
For primary research, interviews are conducted with industry experts/CEOs/Marketing Managers/Sales Managers/VPs/Subject Matter Experts from both demand and supply side to get a 360-degree view of the market. The primary team conducts several interviews based on the complexity of the markets to understand the various market trends and dynamics which makes research more credible and precise.
A typical research interview fulfils the following functions:
- Provides first-hand information on the market size, market trends, growth trends, competitive landscape, and outlook
- Validates and strengthens in-house secondary research findings
- Develops the analysis team’s expertise and market understanding
Primary research involves email interactions and telephone interviews for each market, category, segment, and sub-segment across geographies. The participants who typically take part in such a process include, but are not limited to:
- Industry participants: VPs, business development managers, market intelligence managers and national sales managers
- Outside experts: Valuation experts, research analysts and key opinion leaders specializing in the electronics and semiconductor industry.
Below is the breakup of our primary respondents by company, designation, and region:
Once we receive the confirmation from primary research sources or primary respondents, we finalize the base year market estimation and forecast the data as per the macroeconomic and microeconomic factors assessed during data collection.
- Data Analysis:
Once data is validated through both secondary as well as primary respondents, we finalize the market estimations by hypothesis formulation and factor analysis at regional and country level.
- 3.1 Macro-Economic Factor Analysis:
We analyse macroeconomic indicators such the gross domestic product (GDP), increase in the demand for goods and services across industries, technological advancement, regional economic growth, governmental policies, the influence of COVID-19, PEST analysis, and other aspects. This analysis aids in setting benchmarks for various nations/regions and approximating market splits. Additionally, the general trend of the aforementioned components aid in determining the market's development possibilities.
- 3.2 Country Level Data:
Various factors that are especially aligned to the country are taken into account to determine the market size for a certain area and country, including the presence of vendors, such as headquarters and offices, the country's GDP, demand patterns, and industry growth. To comprehend the market dynamics for the nation, a number of growth variables, inhibitors, application areas, and current market trends are researched. The aforementioned elements aid in determining the country's overall market's growth potential.
- 3.3 Company Profile:
The “Table of Contents” is formulated by listing and analyzing more than 25 - 30 companies operating in the market ecosystem across geographies. However, we profile only 10 companies as a standard practice in our syndicate reports. These 10 companies comprise leading, emerging, and regional players. Nonetheless, our analysis is not restricted to the 10 listed companies, we also analyze other companies present in the market to develop a holistic view and understand the prevailing trends. The “Company Profiles” section in the report covers key facts, business description, products & services, financial information, SWOT analysis, and key developments. The financial information presented is extracted from the annual reports and official documents of the publicly listed companies. Upon collecting the information for the sections of respective companies, we verify them via various primary sources and then compile the data in respective company profiles. The company level information helps us in deriving the base number as well as in forecasting the market size.
- 3.4 Developing Base Number:
Aggregation of sales statistics (2020-2022) and macro-economic factor, and other secondary and primary research insights are utilized to arrive at base number and related market shares for 2022. The data gaps are identified in this step and relevant market data is analyzed, collected from paid primary interviews or databases. On finalizing the base year market size, forecasts are developed on the basis of macro-economic, industry and market growth factors and company level analysis.
- Data Triangulation and Final Review:
The market findings and base year market size calculations are validated from supply as well as demand side. Demand side validations are based on macro-economic factor analysis and benchmarks for respective regions and countries. In case of supply side validations, revenues of major companies are estimated (in case not available) based on industry benchmark, approximate number of employees, product portfolio, and primary interviews revenues are gathered. Further revenue from target product/service segment is assessed to avoid overshooting of market statistics. In case of heavy deviations between supply and demand side values, all thes steps are repeated to achieve synchronization.
We follow an iterative model, wherein we share our research findings with Subject Matter Experts (SME’s) and Key Opinion Leaders (KOLs) until consensus view of the market is not formulated – this model negates any drastic deviation in the opinions of experts. Only validated and universally acceptable research findings are quoted in our reports.
We have important check points that we use to validate our research findings – which we call – data triangulation, where we validate the information, we generate from secondary sources with primary interviews and then we re-validate with our internal data bases and Subject matter experts. This comprehensive model enables us to deliver high quality, reliable data in shortest possible time.
 Get Free Sample For
Get Free Sample For