The Clinical Trial Supplies Market size is projected to reach US$ 8085.87 million by 2031 from US$ 3819.36 million in 2023. The market is expected to register a CAGR of 9.9% in 2024–2031. With the increasing costs of drug discovery, clinical trial supplies are obtaining more importance. In addition, implementing more stringent handling requirements for a type of biopharmaceutical product starting clinical trials and clinical trial supplies strategy needs to be continuously improved. The growth of the global clinical trials supplies market is attributed to key driving factors such as increasing R&D expenditures in the pharmaceutical & biopharmaceutical companies, increase in the number of clinical trials, and higher costs of drug development in developed nations. However, the rising cost of drug development and clinical trials and challenges for clinical trials due to the coronavirus's negative impact are expected to restrain the market growth during the forecast years. The significantly growing chronic diseases across the world are likely to remain a clinical trial supplies market trend.
Clinical Trial Supplies Market Analysis
The growing healthcare sector in developing countries of Asia-Pacific creates better opportunities for the clinical trial supplies management market players to expand their business. The huge patient population in these countries is generating demand for more clinical trials. More clinical trials are conducted in the Asia Pacific than in the US or Europe. This shift is ascribed to low operational costs, large patient recruitment potential, contract research organization's growth, favorable regulatory environment, and better clinical trial capacity and quality. In developed regions such as North America and Europe, ~35% of trials are delayed due to problems in patient recruitment, and one-fifth of the trials are refrained from enrolment due to insufficient subjects. As per the report “Clinical Trials in Asia: A World Health Organization database study,” from 2008 to 2017, the average yearly rise in the number of clinical trials conducted was 41.9% in Iran, 27.1% in Sri Lanka, 23.3% in China, 21.3% in India, 18.4% in Japan, 14.7% in Thailand, 8.4% in Malaysia, and 12.9% in Korea.
The rising number of clinical trial supply market players is expected to drive the global clinical trial supplies market.
Clinical Trial Supplies Market Overview
The clinical trial is an investigation study that defines whether a medical approach, therapy, or device is effective, safe, and useful for human applications. These studies help to find which therapeutic approaches are best for certain diseases. Clinical trial supplies management is necessary for evading overproduction, oversupply, and inventory expiration.
The rising prevalence of chronic diseases boosts the growth of the clinical trial supplies market.
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
Clinical Trial Supplies Market: Strategic Insights
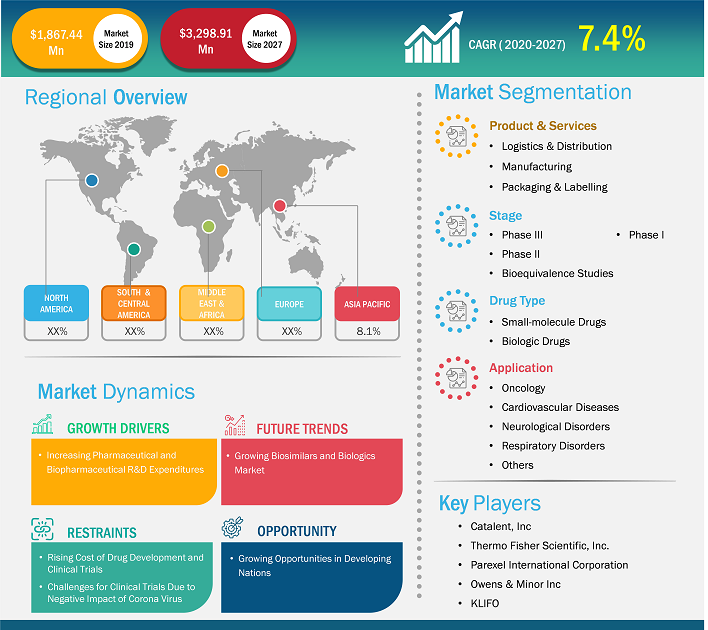
Market Size Value in US$ 1,867.44 Million in 2019 Market Size Value by US$ 3,298.91 Million by 2027 Growth rate CAGR of 7.4% from 2020-2027 Forecast Period 2020-2027 Base Year 2020

Mrinal
Have a question?
Mrinal will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
Clinical Trial Supplies Market: Strategic Insights

| Market Size Value in | US$ 1,867.44 Million in 2019 |
| Market Size Value by | US$ 3,298.91 Million by 2027 |
| Growth rate | CAGR of 7.4% from 2020-2027 |
| Forecast Period | 2020-2027 |
| Base Year | 2020 |

Mrinal
Have a question?
Mrinal will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Clinical Trial Supplies Market Drivers and Opportunities
High Incidence of Chronic Diseases to Favor Market
The high incidence of chronic diseases in Asia is a key factor likely to contribute to the surge in the number of clinical trials in Asia. According to the World Health Organization (WHO), non-communicable diseases cause 55% of the total deaths, ~8 million every year, in the region. In addition, pharmaceutical companies are likely to conduct drug trials for treating gastroesophageal or liver cancer in China or Korea, respectively, as these countries have many patients suffering from these diseases. Moreover, clinical trials in Asia cost ~30–40% less than in the US and the EU as the doctor visits and medical treatments and procedures are less pricey in Asian countries. Such a factor is anticipated to increase the clinical trials, thereby contributing to the clinical trial supplies market growth.
Outsourcing Clinical Trial Services
Outsourcing clinical trials from contract manufacturers and service providers provides sufficient time for pharmaceutical drug manufacturers to develop other drug formulations, maintain frequent and constant communication with other pharmaceutical companies, and prevent risks and other benefits. Companies such as Fisher Clinical Services, Inc., PAREXEL International, and Piramal Pharma Solutions offer logistics and distribution services to pharmaceutical and biopharmaceutical companies. Thus, the factors mentioned above are responsible for the growth of the clinical trial supplies market size.
Clinical Trial Supplies Market Report Segmentation Analysis
Key segments that contributed to the derivation of the clinical trial supplies market analysis are product & services and stage.
- Based on products & services, the market is segmented into manufacturing, packaging & labelling, and logistics & distribution. The logistics & distribution segment held the largest share of the market in 2023; the segment is anticipated to register the highest CAGR in the market during the forecast period. The logistics and distribution segment is estimated to be the largest segment by product and service, owing to the rising contract manufacturing in the pharmaceutical industry. The cost required for the clinical trial process is much higher. Pharmaceutical and biopharmaceutical companies invest a lot in strict handling of pharmaceutical operations during clinical trials. Thus, various pharmaceutical companies outsource their clinical trials to avoid the unnecessary handling costs due to overproduction, oversupply, and inventory expiration. Thus, the factors are responsible for the growth of the clinical trials supply & logistics market.
- Based on stage, the clinical trial supplies market is segmented into phase I, phase II, phase III, and bioequivalence studies. The Phase III segment held the largest share of the market in 2023 and is estimated to register the highest CAGR in the market during the forecast period. Phase III clinical trial stage is done for large patient groups. The phase III clinical trial stage assists in determining the short-term and long-term efficacy of the active pharmaceutical ingredients. Thus, the assessment is done for the formulated drug's total and associated therapeutic values. As many patients are involved in the clinical trials of phase III, it is important to maintain the efficacy, safety, and accuracy of the drug, which otherwise may result in adverse effects of the drugs in many patients registered for the phase III clinical trials. Shertech Manufacturing is one such company that offers phase III clinical trial services, and it offers comprehensive, definitive data related to efficacy and side effects to its customers. It also assists in complying with FDA standards that further assist in introducing the drug into the market and completing the required licensing applications. Thus, pain management contributes to the clinical trial supplies market and is expected to continue the trend during the forecast period.
Clinical Trial Supplies Market Share Analysis by Geography
The geographic scope of the clinical trial supplies market report is mainly divided into five regions: North America, Asia Pacific, Europe, the Middle East & Africa, and South & Central America.
Based on geography, the clinical trials supplies market is divided into five key regions: North America, Europe, Asia Pacific, South & Central America, and Middle East & Africa. The North American, clinical trials supplies market has been analyzed based on three major countries — the US, Canada, and Mexico. The US clinical trials supply market is estimated to hold the largest market share during the forecast period. The US clinical trial supplies market growth is attributed to the presence of market players in the region that offer manufacturing, storage, logistics, and other services, which is likely to enhance the market growth in the country. For instance, Alderley Analytical, Almac, and others are well-known manufacturing organizations offering a wide range of integrated services to more than 600 pharmaceutical and biotech companies. Additionally, the rise in demand for services and medicines in areas of orphan and rare diseases is expected to encourage drug manufacturers to develop smart medicines and recruit patients for clinical trials. The increasing urge for the development of new drugs in the country is also expected to increase the number of industry entrants by keeping up with the fast pace of the pharmaceutical industry.
Clinical Trial Supplies Market Report Scope
Clinical Trial Supplies Market News and Recent Developments
The clinical trial supplies market is evaluated by gathering qualitative and quantitative data post primary and secondary research, which includes important corporate publications, association data, and databases. The following is a list of developments in the market for innovations, business expansion, and strategies:
- In May 2020, Sharp, part of UDG Healthcare, purchased a pharmaceutical packaging facility from Quality Packaging Specialists International (QPSI) in the US. Situated in Macungie, Pennsylvania, the 160,000ft² facility provides primary and secondary pharmaceutical packaging services to its customers. The Macungie facility provides bottling, blistering, vial labeling, medical device kitting, and sterilization services (Source: Sharp, Press Release)
- In April 2021, Catalent added cryogenic capabilities at its Philadelphia clinical supply services facility. This expansion helped increase Catalent's capabilities in gene therapies, packaging, labeling, and distribution of cryogenic materials for clinical trials (Source: Catalent, Inc., Press Release)
Clinical Trial Supplies Market Report Coverage and Deliverables
The “Clinical Trial Supplies Market Size and Forecast (2021–2031)” report provides a detailed analysis of the market covering the following areas:
- Clinical Trial SuppliesMarket size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Market dynamics such as drivers, restraints, and key opportunities
- Clinical Trial Supplies Market trends
- Detailed PEST/Porter’s Five Forces and SWOT analysis
- Clinical Trial Supplies market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Clinical Trial SuppliesIndustry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments
- Detailed company profiles

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Product & Service ; Stage ; Drug Type ; Application , and Geography

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, Saudi Arabia, South Africa, South Korea, Spain, United Arab Emirates, United Kingdom, United States
The Insight Partners performs research in 4 major stages: Data Collection & Secondary Research, Primary Research, Data Analysis and Data Triangulation & Final Review.
- Data Collection and Secondary Research:
As a market research and consulting firm operating from a decade, we have published many reports and advised several clients across the globe. First step for any study will start with an assessment of currently available data and insights from existing reports. Further, historical and current market information is collected from Investor Presentations, Annual Reports, SEC Filings, etc., and other information related to company’s performance and market positioning are gathered from Paid Databases (Factiva, Hoovers, and Reuters) and various other publications available in public domain.
Several associations trade associates, technical forums, institutes, societies and organizations are accessed to gain technical as well as market related insights through their publications such as research papers, blogs and press releases related to the studies are referred to get cues about the market. Further, white papers, journals, magazines, and other news articles published in the last 3 years are scrutinized and analyzed to understand the current market trends.
- Primary Research:
The primarily interview analysis comprise of data obtained from industry participants interview and answers to survey questions gathered by in-house primary team.
For primary research, interviews are conducted with industry experts/CEOs/Marketing Managers/Sales Managers/VPs/Subject Matter Experts from both demand and supply side to get a 360-degree view of the market. The primary team conducts several interviews based on the complexity of the markets to understand the various market trends and dynamics which makes research more credible and precise.
A typical research interview fulfils the following functions:
- Provides first-hand information on the market size, market trends, growth trends, competitive landscape, and outlook
- Validates and strengthens in-house secondary research findings
- Develops the analysis team’s expertise and market understanding
Primary research involves email interactions and telephone interviews for each market, category, segment, and sub-segment across geographies. The participants who typically take part in such a process include, but are not limited to:
- Industry participants: VPs, business development managers, market intelligence managers and national sales managers
- Outside experts: Valuation experts, research analysts and key opinion leaders specializing in the electronics and semiconductor industry.
Below is the breakup of our primary respondents by company, designation, and region:

Once we receive the confirmation from primary research sources or primary respondents, we finalize the base year market estimation and forecast the data as per the macroeconomic and microeconomic factors assessed during data collection.
- Data Analysis:
Once data is validated through both secondary as well as primary respondents, we finalize the market estimations by hypothesis formulation and factor analysis at regional and country level.
- 3.1 Macro-Economic Factor Analysis:
We analyse macroeconomic indicators such the gross domestic product (GDP), increase in the demand for goods and services across industries, technological advancement, regional economic growth, governmental policies, the influence of COVID-19, PEST analysis, and other aspects. This analysis aids in setting benchmarks for various nations/regions and approximating market splits. Additionally, the general trend of the aforementioned components aid in determining the market's development possibilities.
- 3.2 Country Level Data:
Various factors that are especially aligned to the country are taken into account to determine the market size for a certain area and country, including the presence of vendors, such as headquarters and offices, the country's GDP, demand patterns, and industry growth. To comprehend the market dynamics for the nation, a number of growth variables, inhibitors, application areas, and current market trends are researched. The aforementioned elements aid in determining the country's overall market's growth potential.
- 3.3 Company Profile:
The “Table of Contents” is formulated by listing and analyzing more than 25 - 30 companies operating in the market ecosystem across geographies. However, we profile only 10 companies as a standard practice in our syndicate reports. These 10 companies comprise leading, emerging, and regional players. Nonetheless, our analysis is not restricted to the 10 listed companies, we also analyze other companies present in the market to develop a holistic view and understand the prevailing trends. The “Company Profiles” section in the report covers key facts, business description, products & services, financial information, SWOT analysis, and key developments. The financial information presented is extracted from the annual reports and official documents of the publicly listed companies. Upon collecting the information for the sections of respective companies, we verify them via various primary sources and then compile the data in respective company profiles. The company level information helps us in deriving the base number as well as in forecasting the market size.
- 3.4 Developing Base Number:
Aggregation of sales statistics (2020-2022) and macro-economic factor, and other secondary and primary research insights are utilized to arrive at base number and related market shares for 2022. The data gaps are identified in this step and relevant market data is analyzed, collected from paid primary interviews or databases. On finalizing the base year market size, forecasts are developed on the basis of macro-economic, industry and market growth factors and company level analysis.
- Data Triangulation and Final Review:
The market findings and base year market size calculations are validated from supply as well as demand side. Demand side validations are based on macro-economic factor analysis and benchmarks for respective regions and countries. In case of supply side validations, revenues of major companies are estimated (in case not available) based on industry benchmark, approximate number of employees, product portfolio, and primary interviews revenues are gathered. Further revenue from target product/service segment is assessed to avoid overshooting of market statistics. In case of heavy deviations between supply and demand side values, all thes steps are repeated to achieve synchronization.
We follow an iterative model, wherein we share our research findings with Subject Matter Experts (SME’s) and Key Opinion Leaders (KOLs) until consensus view of the market is not formulated – this model negates any drastic deviation in the opinions of experts. Only validated and universally acceptable research findings are quoted in our reports.
We have important check points that we use to validate our research findings – which we call – data triangulation, where we validate the information, we generate from secondary sources with primary interviews and then we re-validate with our internal data bases and Subject matter experts. This comprehensive model enables us to deliver high quality, reliable data in shortest possible time.
 Get Free Sample For
Get Free Sample For